Team:TUDelft/10 August 2009
Lab Notebook
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
10 August 2009
Sriram
On friday we discussed how to do our project with backup plans. Daniel explained there is another way to make assembly of negative cascade plasmid. So we have a back up plan. I continued with the assembly by preparing cultures for miniprep tomorrow.
Calin
Knockout plasmids pKD3, pKD4, pKD46 arrived. Plates and tubes made for all with Amp. pKD46 kept at 30 C.
trbK plasmid from BaseClear was digested first with HindIII (no BSA) and after with BamHI (1 hr incubation, 20 min inactivation). pSB1A3 was digested with E+P (6uL DNA, 36.5uL H2O). pSB4A5 was digested with E+P (6.5uL DNA, 36uL H2O).
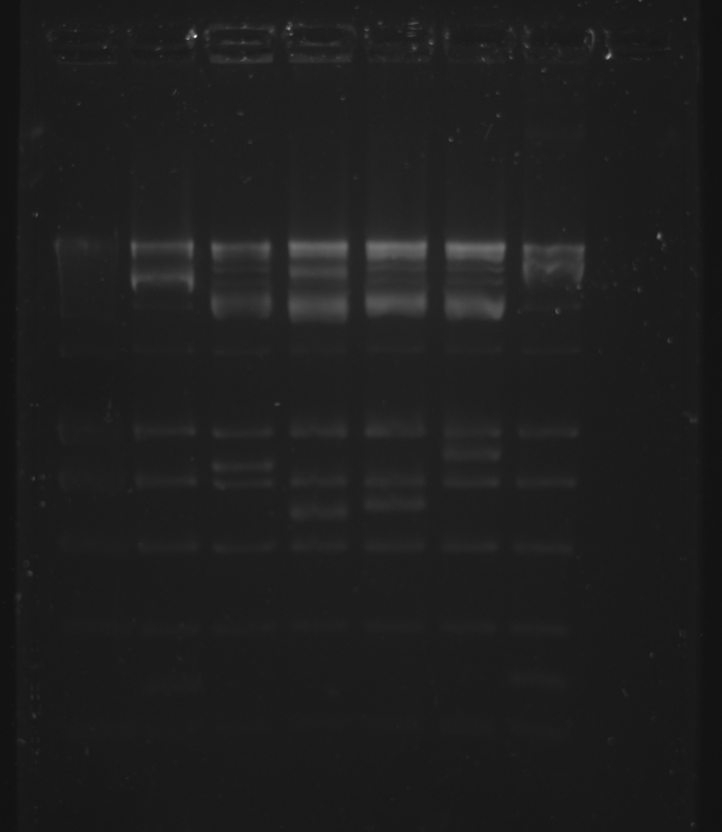
2% Gel was run, Ladder was accidentally used instead of dye.
| Well | Part | Expected Plasmid Size | Status |
| 1 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✔ | |
| 2 | trbK BamHI + HindIII digest | 282 | ✔ |
| 3 | pSB1A3 E+P digest | 2114 | ✔ |
| 4 | pSB4A5 E+P digest | 3395 | ✔ |
| 5 | pSB1C3 E+P digest | 2072 | ✔ |
| 6 | GFP-gen E+S digest | 878 | ✔ |
| 7 | oriT-R X+P digest | 278 | ✔ |
The appropriate bands were cut out with a scalpel under the blue light imager and the QIAGEN Gel Extraction kit was used.
Small colonies on the pTet-RBS plate grew larger by end of the day. Daniel re-transformed pTet-RBS.
5 mL tubes were made by Sriram for:
- Assembly CA
- pTet-RBS
Also a 50 mL R751 culture with 2 x TRI was made.
Daniel
I spent the previous weeks planning and designing the time-delay genetic circuit. Besides, I constructed the "algorithm" for the key/lock generator which Calin transformed in a Java script. After several non successfully attempts to construct the delay circuit (in all modalities), the team decided to change the strategy which I planned and will start today. Also, I will start the lab work towards the characterization of two locks and keys resulted from our "algorithm" for the weak RBS (B0031) and the medium RBS (B0032)
Next it is listed the lab work I did today:
- Rehydratation of biobricks: E0240, B0031, J04630, J123002 and I13507.
- Transformation of biobricks into chemically competent E. coli by heat shock.
- Plating in respective antibiotic selection.
 "
"