Team:TUDelft/19 August 2009
From 2009.igem.org
(→Tim Weenink) |
(→19 August 2009) |
||
| (21 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
='''19 August 2009'''= | ='''19 August 2009'''= | ||
| + | ===Sriram=== | ||
| + | Today I did minipreps of all the biobricks I cultures yesterday. then again I continued with the assembly by digesting all the parts with the SpeI available in our lab. I am not very confident of it since the expiry date shows somewhere in 2008. So I thought let me continue the work properly after discussing with supervisors on friday because Daniel's backup plan biobricks are working very well. | ||
===Tim Weenink=== | ===Tim Weenink=== | ||
| Line 9: | Line 11: | ||
After yesterdays horrible discovery that the I-SceI Homing Endonuclease we got from MIT was actually T4 DNA ligase we had to adjust our cloning strategy. The more primitive part without promoter was found to be the correct one, except that is was assembled with part !A instead of p(LacI). So we did that assembly again today. With p(LacI) from the delay team upstream, *T1 (downstream) and backbone pSB1C3 (also from the delay team). Both RbCl chemically comp and EC electrocomp cells were transformed. | After yesterdays horrible discovery that the I-SceI Homing Endonuclease we got from MIT was actually T4 DNA ligase we had to adjust our cloning strategy. The more primitive part without promoter was found to be the correct one, except that is was assembled with part !A instead of p(LacI). So we did that assembly again today. With p(LacI) from the delay team upstream, *T1 (downstream) and backbone pSB1C3 (also from the delay team). Both RbCl chemically comp and EC electrocomp cells were transformed. | ||
| - | |||
| - | https://2009.igem.org/Image: | + | ===Saeed=== |
| + | [[Image:Saeed19082009wiki.jpg|thumb|center|250px]] | ||
| + | |||
| + | {| border="1" align="center" | ||
| + | | lane || Part || Expected Plasmid Size || Status | ||
| + | |- align="center" | ||
| + | | 1 || [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] || || | ||
| + | |- align="center" | ||
| + | | 2 || !Fr1n upstream || 1900 || ok, but not needed | ||
| + | |- align="center" | ||
| + | | 3 || !Fr1n downstream || 1900 || ok | ||
| + | |- align="center" | ||
| + | | 4 || destination plasmid || 3000 || ok | ||
| + | |} | ||
| + | |||
| + | !Fr1n downstream will be purified from the gel by gel extraction and used for the new stategy as Tim mentioned before. | ||
| + | |||
| + | ===Daniel=== | ||
| + | |||
| + | Digestion of biobricks: | ||
| + | |||
| + | [[Image:gel180809.jpg|550px]] | ||
| + | |||
| + | {| border="1" align="center" | ||
| + | | Well || Biobrick || Expected Plasmid Size || Status | ||
| + | |- align="center" | ||
| + | | 1 || [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] || || | ||
| + | |- align="center" | ||
| + | | 2 || C0051 || 750 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 3 || I13507 || 861 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 4 || P0440 || 840 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 5 || I13504 || 875 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 6 || J04630 || 857 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 7 || R0051 || 44 || too small | ||
| + | |- align="center" | ||
| + | | 8 || B0015 || 129 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 9 || J13002 || 74 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 10 || pSB1C3 || 2072 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 11 || pSB1AK3 || 3426 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 12 || pSB1A3 || 2157 || <font color=limegreen>✔</font> | ||
| + | |- align="center" | ||
| + | | 13 || CB || || | ||
| + | |- align="center" | ||
| + | | 14 || CC || || | ||
| + | |- align="center" | ||
| + | | 15 || [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] || || | ||
| + | |} | ||
| + | |||
| + | Assemblies 1A, 2A, 3A, 4A, 5A and 6A | ||
| + | |||
| + | Transformation and culture in plates and tubes | ||
| + | |||
| + | ===Calin=== | ||
| + | |||
| + | Good results on the conjugation test. All plates were imaged on the safe imager with the 5 Megapixel camera. Colony counting was done with [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1770926 Clono-Counter]. | ||
| + | |||
| + | For the trbK conjugation test: | ||
| + | |||
| + | {| border="1" align="center" | ||
| + | |- | ||
| + | | <b>Set 1</b> | ||
| + | |- align="center" | ||
| + | | Plate ID || Antibiotics || Dilution || # Colonies | ||
| + | |- align="center" | ||
| + | | D1tr || TRI || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | D2tr || TRI || 10<sup>-1</sup>|| overgrown | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-trbK_D3tr.jpeg D3tr] || TRI || 10<sup>-2</sup>|| <font color=green><B>4450</b></font> | ||
| + | |- align="center" | ||
| + | | D4tr || TRI || 10<sup>-3</sup>|| 714 | ||
| + | |- align="center" | ||
| + | | D5tr || TRI || 10<sup>-4</sup>|| 86 | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-trbK_T1tr.jpeg T1tr] || TRI + CAM || 10<sup>0</sup> || <font color=green><B>2</b></font> | ||
| + | |- align="center" | ||
| + | | T2tr || TRI + CAM || 10<sup>-1</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | T3tr || TRI + CAM || 10<sup>-2</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | T4tr || TRI + CAM || 10<sup>-3</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | T5tr || TRI + CAM || 10<sup>-4</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | R1tr || CAM || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | R2tr || CAM || 10<sup>-1</sup>|| 8636 | ||
| + | |} | ||
| + | |||
| + | <br><br> | ||
| + | {| border="1" align="center" | ||
| + | |- | ||
| + | | <b>Set 2</b> | ||
| + | |- align="center" | ||
| + | | Plate ID || Antibiotics || Dilution || # Colonies | ||
| + | |- align="center" | ||
| + | | D6tr || TRI || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | D7tr || TRI || 10<sup>-1</sup>|| overgrown | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-trbK_D8tr.jpeg D8tr] || TRI || 10<sup>-2</sup>|| <font color=green><B>6930</b></font> | ||
| + | |- align="center" | ||
| + | | D9tr || TRI || 10<sup>-3</sup>|| 720 | ||
| + | |- align="center" | ||
| + | | D10tr || TRI || 10<sup>-4</sup>|| 81 | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-trbK_T6tr.jpeg T6tr] || TRI + CAM || 10<sup>0</sup> || <font color=green><B>2</b></font> | ||
| + | |- align="center" | ||
| + | | T7tr || TRI + CAM || 10<sup>-1</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | T8tr || TRI + CAM || 10<sup>-2</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | T9tr || TRI + CAM || 10<sup>-3</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | T10tr || TRI + CAM || 10<sup>-4</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | R3tr || CAM || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | R4tr || CAM || 10<sup>-1</sup>|| 6333 | ||
| + | |} | ||
| + | |||
| + | also 3 positive control plates with no TrbK expression. | ||
| + | |||
| + | <br> | ||
| + | {| border="1" align="center" | ||
| + | |- | ||
| + | | <b>Results</b> | ||
| + | |- align="center" | ||
| + | | Set # || Conjugation Efficinecy | ||
| + | |- align="center" | ||
| + | | 1 || 4.49E-6 | ||
| + | |- align="center" | ||
| + | | 2 || 2.89E-6 | ||
| + | |- align="center" | ||
| + | | control || 0.031 | ||
| + | |- align="center" | ||
| + | |} | ||
| + | |||
| + | This successfully shows a reduction in the conjugation efficiency by four orders of magnitude when the entry exclusion protein is expressed. | ||
| + | |||
| + | |||
| + | For the oriTR conjugation test: | ||
| + | |||
| + | {| border="1" align="center" | ||
| + | |- | ||
| + | | <b>Set 1</b> | ||
| + | |- align="center" | ||
| + | | Plate ID || Antibiotics || Dilution || # Colonies | ||
| + | |- align="center" | ||
| + | | D1ori || TRI + CAM || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | D2ori || TRI + CAM || 10<sup>-1</sup>|| overgrown | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-oriTR_D3oriGFP.jpeg D3ori] || TRI + CAM || 10<sup>-2</sup>|| <font color=green><B>2939</b></font> | ||
| + | |- align="center" | ||
| + | | D4ori || TRI + CAM || 10<sup>-3</sup>|| 470 | ||
| + | |- align="center" | ||
| + | | D5ori || TRI + CAM || 10<sup>-4</sup>|| 48 | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-oriTR_Ts1oriGFP.jpeg Ts1ori] || AMP + CAM || 10<sup>0</sup> || <font color=green><B>520</b></font> | ||
| + | |- align="center" | ||
| + | | Ts2ori || AMP + CAM || 10<sup>-1</sup>|| 1 | ||
| + | |- align="center" | ||
| + | | Ts3ori || AMP + CAM || 10<sup>-2</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | Ts4ori || AMP + CAM || 10<sup>-3</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | Ts5ori || AMP + CAM || 10<sup>-4</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-oriTR_tl1.jpeg TL1ori] || TRI + AMP || 10<sup>0</sup> || <font color=green><B>4092</b></font> | ||
| + | |- align="center" | ||
| + | | TL2ori || TRI + AMP || 10<sup>-1</sup>|| 802 | ||
| + | |- align="center" | ||
| + | | TL3ori || TRI + AMP || 10<sup>-2</sup>|| 94 | ||
| + | |- align="center" | ||
| + | | TL4ori || TRI + AMP || 10<sup>-3</sup>|| 7 | ||
| + | |- align="center" | ||
| + | | TL5ori || TRI + AMP || 10<sup>-4</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | R1ori || AMP || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | R2ori || AMP || 10<sup>-1</sup>|| 5187 | ||
| + | |} | ||
| + | |||
| + | <br><br> | ||
| + | {| border="1" align="center" | ||
| + | |- | ||
| + | | <b>Set 2</b> | ||
| + | |- align="center" | ||
| + | | Plate ID || Antibiotics || Dilution || # Colonies | ||
| + | |- align="center" | ||
| + | | D6ori || TRI + CAM || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | D7ori || TRI + CAM || 10<sup>-1</sup>|| overgrown | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-oriTR_D8oriGFP.jpeg D8ori] || TRI + CAM || 10<sup>-2</sup>|| <font color=green><B>4950</b></font> | ||
| + | |- align="center" | ||
| + | | D9ori || TRI + CAM || 10<sup>-3</sup>|| 1066 | ||
| + | |- align="center" | ||
| + | | D10ori || TRI + CAM || 10<sup>-4</sup>|| 114 | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-oriTR_Ts6oriGFP.jpeg Ts6ori] || AMP + CAM || 10<sup>0</sup> || <font color=green><B>1105</b></font> | ||
| + | |- align="center" | ||
| + | | Ts7ori || AMP + CAM || 10<sup>-1</sup>|| 12 | ||
| + | |- align="center" | ||
| + | | Ts8ori || AMP + CAM || 10<sup>-2</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | Ts9ori || AMP + CAM || 10<sup>-3</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | Ts10ori || AMP + CAM || 10<sup>-4</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | TL6ori || TRI + AMP || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-oriTR_tl7.jpeg TL7ori] || TRI + AMP || 10<sup>-1</sup>|| <font color=green><B>2251</b></font> | ||
| + | |- align="center" | ||
| + | | TL8ori || TRI + AMP || 10<sup>-2</sup>|| 352 | ||
| + | |- align="center" | ||
| + | | TL9ori || TRI + AMP || 10<sup>-3</sup>|| 39 | ||
| + | |- align="center" | ||
| + | | TL10ori || TRI + AMP || 10<sup>-4</sup>|| 7 | ||
| + | |- align="center" | ||
| + | | R3ori || AMP || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | R4ori || AMP || 10<sup>-1</sup>|| 3961 | ||
| + | |} | ||
| + | |||
| + | <br><br> | ||
| + | {| border="1" align="center" | ||
| + | |- | ||
| + | | <b>Set 3</b> | ||
| + | |- align="center" | ||
| + | | Plate ID || Antibiotics || Dilution || # Colonies | ||
| + | |- align="center" | ||
| + | | D11ori || TRI + CAM || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | D12ori || TRI + CAM || 10<sup>-1</sup>|| overgrown | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-oriTR_D13oriGFP.jpeg D13ori] || TRI + CAM || 10<sup>-2</sup>|| <font color=green><B>4180</b></font> | ||
| + | |- align="center" | ||
| + | | D14ori || TRI + CAM || 10<sup>-3</sup>|| 737 | ||
| + | |- align="center" | ||
| + | | D15ori || TRI + CAM || 10<sup>-4</sup>|| 46 | ||
| + | |- align="center" | ||
| + | | Ts11ori || AMP + CAM || 10<sup>0</sup> || bad plate | ||
| + | |- align="center" | ||
| + | | Ts12ori || AMP + CAM || 10<sup>-1</sup>|| 3 | ||
| + | |- align="center" | ||
| + | | Ts13ori || AMP + CAM || 10<sup>-2</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | Ts14ori || AMP + CAM || 10<sup>-3</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | Ts15ori || AMP + CAM || 10<sup>-4</sup>|| 0 | ||
| + | |- align="center" | ||
| + | | TL11ori || TRI + AMP || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | [https://2009.igem.org/Image:Conjugation-aug18-oriTR_tl12.jpeg TL12ori] || TRI + AMP || 10<sup>-1</sup>|| <font color=green><B>1436</b></font> | ||
| + | |- align="center" | ||
| + | | TL13ori || TRI + AMP || 10<sup>-2</sup>|| 172 | ||
| + | |- align="center" | ||
| + | | TL14ori || TRI + AMP || 10<sup>-3</sup>|| 18 | ||
| + | |- align="center" | ||
| + | | TL15ori || TRI + AMP || 10<sup>-4</sup>|| 3 | ||
| + | |- align="center" | ||
| + | | R5ori || AMP || 10<sup>0</sup> || overgrown | ||
| + | |- align="center" | ||
| + | | R6ori || AMP || 10<sup>-1</sup>|| 3679 | ||
| + | |} | ||
| + | |||
| + | <br> | ||
| + | {| border="1" align="center" | ||
| + | |- | ||
| + | | <b>Results</b> | ||
| + | |- align="center" | ||
| + | | Set # || Conjugation Efficiency for Plasmid 1 || Conjugation Efficiency for wild R751 | ||
| + | |- align="center" | ||
| + | | 1 || 0.00177 || 0.0141 | ||
| + | |- align="center" | ||
| + | | 2 || 0.00224 || 0.0476 | ||
| + | |- align="center" | ||
| + | | 3|| n/a || 0.0356 | ||
| + | |- align="center" | ||
| + | |} | ||
| + | |||
| + | This shows that Conjugation Plasmid 1 is successfully transmitted but with a lower efficiency in the presence of R751. | ||
| + | |||
| + | |||
| + | Attempted 2nd knockout | ||
| + | |||
| + | Made 4 0.5xKAN plates, 2 0.1xKAN plates, 2 1xTRI plates and 2 5mL tubes with 0.5xKAN. | ||
| + | |||
| + | Started centrifuging when R751 was at OD 0.507. | ||
| + | |||
| + | Used 7 μL oriTR_PCR_KO vector for oriTR electroporation and 12 μL trbK_KO_PCR vector for trbK electroporation. Both time constants 3.7. In incubator at 4:13 pm. | ||
| + | |||
| + | Modifications made to protocol: | ||
| + | * Arabinose added the night before | ||
| + | * used 4 mL from the 5 mL culture, higher density of cells in elctroporation | ||
| + | * used 10% glycerol in the last spin down step | ||
| + | |||
| + | {{Template:TUDelftiGEM2009_end}} | ||
Latest revision as of 10:09, 20 October 2009
Lab Notebook
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
19 August 2009
Sriram
Today I did minipreps of all the biobricks I cultures yesterday. then again I continued with the assembly by digesting all the parts with the SpeI available in our lab. I am not very confident of it since the expiry date shows somewhere in 2008. So I thought let me continue the work properly after discussing with supervisors on friday because Daniel's backup plan biobricks are working very well.
Tim Weenink
After yesterdays horrible discovery that the I-SceI Homing Endonuclease we got from MIT was actually T4 DNA ligase we had to adjust our cloning strategy. The more primitive part without promoter was found to be the correct one, except that is was assembled with part !A instead of p(LacI). So we did that assembly again today. With p(LacI) from the delay team upstream, *T1 (downstream) and backbone pSB1C3 (also from the delay team). Both RbCl chemically comp and EC electrocomp cells were transformed.
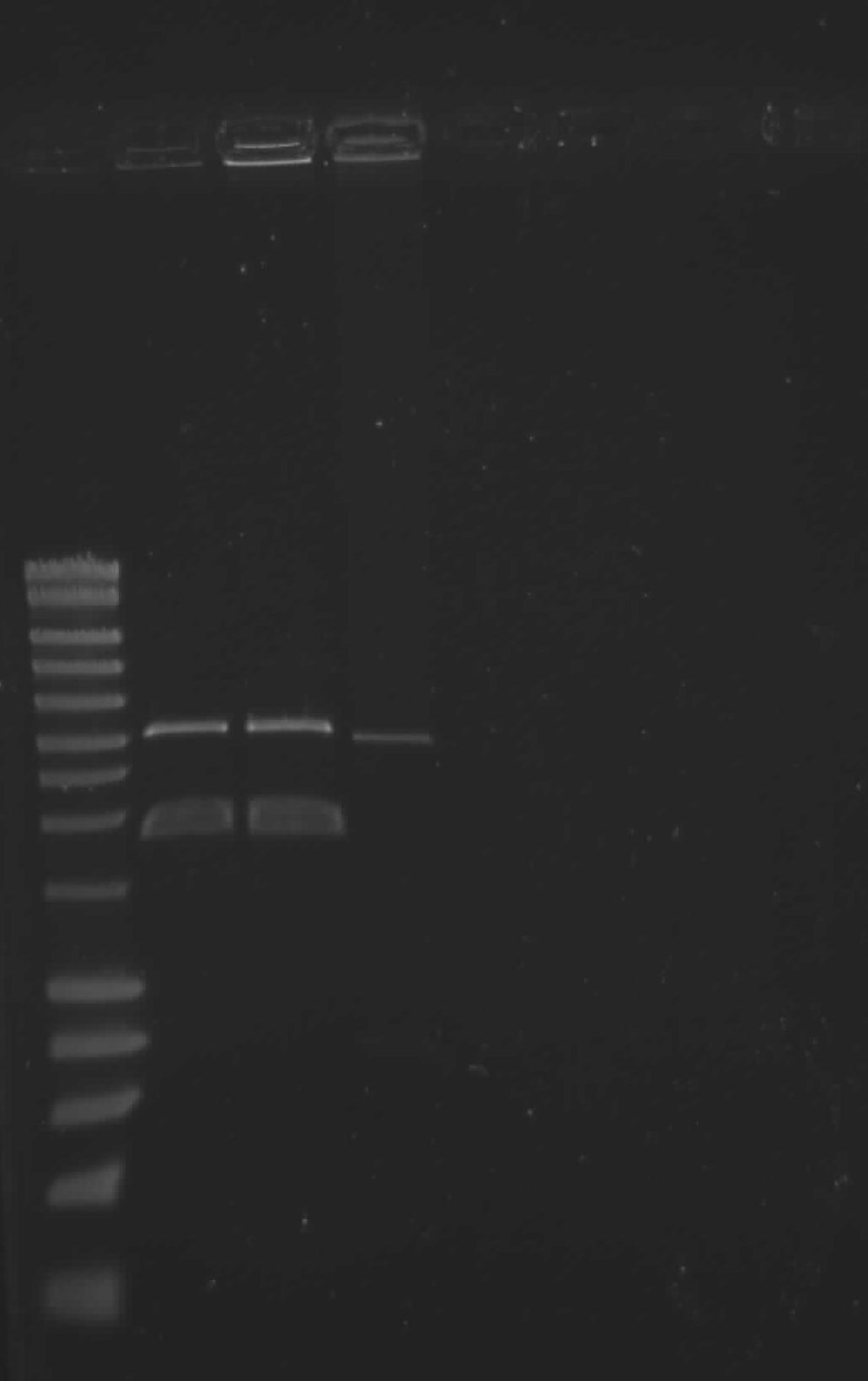
Saeed
| lane | Part | Expected Plasmid Size | Status |
| 1 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ||
| 2 | !Fr1n upstream | 1900 | ok, but not needed |
| 3 | !Fr1n downstream | 1900 | ok |
| 4 | destination plasmid | 3000 | ok |
!Fr1n downstream will be purified from the gel by gel extraction and used for the new stategy as Tim mentioned before.
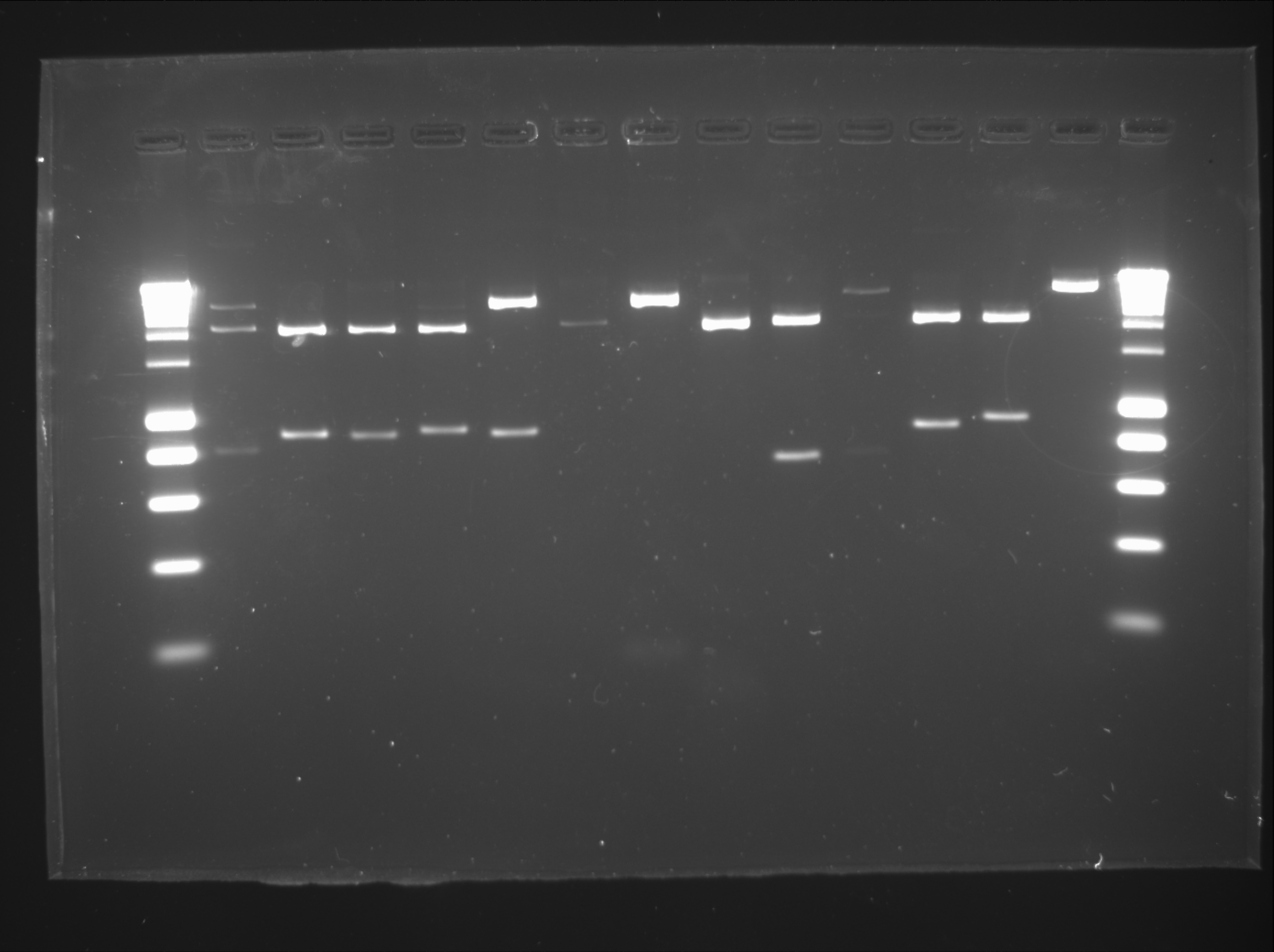
Daniel
Digestion of biobricks:
| Well | Biobrick | Expected Plasmid Size | Status |
| 1 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ||
| 2 | C0051 | 750 | ✔ |
| 3 | I13507 | 861 | ✔ |
| 4 | P0440 | 840 | ✔ |
| 5 | I13504 | 875 | ✔ |
| 6 | J04630 | 857 | ✔ |
| 7 | R0051 | 44 | too small |
| 8 | B0015 | 129 | ✔ |
| 9 | J13002 | 74 | ✔ |
| 10 | pSB1C3 | 2072 | ✔ |
| 11 | pSB1AK3 | 3426 | ✔ |
| 12 | pSB1A3 | 2157 | ✔ |
| 13 | CB | ||
| 14 | CC | ||
| 15 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] |
Assemblies 1A, 2A, 3A, 4A, 5A and 6A
Transformation and culture in plates and tubes
Calin
Good results on the conjugation test. All plates were imaged on the safe imager with the 5 Megapixel camera. Colony counting was done with [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1770926 Clono-Counter].
For the trbK conjugation test:
| Set 1 | |||
| Plate ID | Antibiotics | Dilution | # Colonies |
| D1tr | TRI | 100 | overgrown |
| D2tr | TRI | 10-1 | overgrown |
| D3tr | TRI | 10-2 | 4450 |
| D4tr | TRI | 10-3 | 714 |
| D5tr | TRI | 10-4 | 86 |
| T1tr | TRI + CAM | 100 | 2 |
| T2tr | TRI + CAM | 10-1 | 0 |
| T3tr | TRI + CAM | 10-2 | 0 |
| T4tr | TRI + CAM | 10-3 | 0 |
| T5tr | TRI + CAM | 10-4 | 0 |
| R1tr | CAM | 100 | overgrown |
| R2tr | CAM | 10-1 | 8636 |
| Set 2 | |||
| Plate ID | Antibiotics | Dilution | # Colonies |
| D6tr | TRI | 100 | overgrown |
| D7tr | TRI | 10-1 | overgrown |
| D8tr | TRI | 10-2 | 6930 |
| D9tr | TRI | 10-3 | 720 |
| D10tr | TRI | 10-4 | 81 |
| T6tr | TRI + CAM | 100 | 2 |
| T7tr | TRI + CAM | 10-1 | 0 |
| T8tr | TRI + CAM | 10-2 | 0 |
| T9tr | TRI + CAM | 10-3 | 0 |
| T10tr | TRI + CAM | 10-4 | 0 |
| R3tr | CAM | 100 | overgrown |
| R4tr | CAM | 10-1 | 6333 |
also 3 positive control plates with no TrbK expression.
| Results | |
| Set # | Conjugation Efficinecy |
| 1 | 4.49E-6 |
| 2 | 2.89E-6 |
| control | 0.031 |
This successfully shows a reduction in the conjugation efficiency by four orders of magnitude when the entry exclusion protein is expressed.
For the oriTR conjugation test:
| Set 1 | |||
| Plate ID | Antibiotics | Dilution | # Colonies |
| D1ori | TRI + CAM | 100 | overgrown |
| D2ori | TRI + CAM | 10-1 | overgrown |
| D3ori | TRI + CAM | 10-2 | 2939 |
| D4ori | TRI + CAM | 10-3 | 470 |
| D5ori | TRI + CAM | 10-4 | 48 |
| Ts1ori | AMP + CAM | 100 | 520 |
| Ts2ori | AMP + CAM | 10-1 | 1 |
| Ts3ori | AMP + CAM | 10-2 | 0 |
| Ts4ori | AMP + CAM | 10-3 | 0 |
| Ts5ori | AMP + CAM | 10-4 | 0 |
| TL1ori | TRI + AMP | 100 | 4092 |
| TL2ori | TRI + AMP | 10-1 | 802 |
| TL3ori | TRI + AMP | 10-2 | 94 |
| TL4ori | TRI + AMP | 10-3 | 7 |
| TL5ori | TRI + AMP | 10-4 | 0 |
| R1ori | AMP | 100 | overgrown |
| R2ori | AMP | 10-1 | 5187 |
| Set 2 | |||
| Plate ID | Antibiotics | Dilution | # Colonies |
| D6ori | TRI + CAM | 100 | overgrown |
| D7ori | TRI + CAM | 10-1 | overgrown |
| D8ori | TRI + CAM | 10-2 | 4950 |
| D9ori | TRI + CAM | 10-3 | 1066 |
| D10ori | TRI + CAM | 10-4 | 114 |
| Ts6ori | AMP + CAM | 100 | 1105 |
| Ts7ori | AMP + CAM | 10-1 | 12 |
| Ts8ori | AMP + CAM | 10-2 | 0 |
| Ts9ori | AMP + CAM | 10-3 | 0 |
| Ts10ori | AMP + CAM | 10-4 | 0 |
| TL6ori | TRI + AMP | 100 | overgrown |
| TL7ori | TRI + AMP | 10-1 | 2251 |
| TL8ori | TRI + AMP | 10-2 | 352 |
| TL9ori | TRI + AMP | 10-3 | 39 |
| TL10ori | TRI + AMP | 10-4 | 7 |
| R3ori | AMP | 100 | overgrown |
| R4ori | AMP | 10-1 | 3961 |
| Set 3 | |||
| Plate ID | Antibiotics | Dilution | # Colonies |
| D11ori | TRI + CAM | 100 | overgrown |
| D12ori | TRI + CAM | 10-1 | overgrown |
| D13ori | TRI + CAM | 10-2 | 4180 |
| D14ori | TRI + CAM | 10-3 | 737 |
| D15ori | TRI + CAM | 10-4 | 46 |
| Ts11ori | AMP + CAM | 100 | bad plate |
| Ts12ori | AMP + CAM | 10-1 | 3 |
| Ts13ori | AMP + CAM | 10-2 | 0 |
| Ts14ori | AMP + CAM | 10-3 | 0 |
| Ts15ori | AMP + CAM | 10-4 | 0 |
| TL11ori | TRI + AMP | 100 | overgrown |
| TL12ori | TRI + AMP | 10-1 | 1436 |
| TL13ori | TRI + AMP | 10-2 | 172 |
| TL14ori | TRI + AMP | 10-3 | 18 |
| TL15ori | TRI + AMP | 10-4 | 3 |
| R5ori | AMP | 100 | overgrown |
| R6ori | AMP | 10-1 | 3679 |
| Results | ||
| Set # | Conjugation Efficiency for Plasmid 1 | Conjugation Efficiency for wild R751 |
| 1 | 0.00177 | 0.0141 |
| 2 | 0.00224 | 0.0476 |
| 3 | n/a | 0.0356 |
This shows that Conjugation Plasmid 1 is successfully transmitted but with a lower efficiency in the presence of R751.
Attempted 2nd knockout
Made 4 0.5xKAN plates, 2 0.1xKAN plates, 2 1xTRI plates and 2 5mL tubes with 0.5xKAN.
Started centrifuging when R751 was at OD 0.507.
Used 7 μL oriTR_PCR_KO vector for oriTR electroporation and 12 μL trbK_KO_PCR vector for trbK electroporation. Both time constants 3.7. In incubator at 4:13 pm.
Modifications made to protocol:
- Arabinose added the night before
- used 4 mL from the 5 mL culture, higher density of cells in elctroporation
- used 10% glycerol in the last spin down step
 "
"