Team:DTU Denmark/results
From 2009.igem.org
| Line 244: | Line 244: | ||
<font size="3"><b>Extraction of p241i and cloning</b></font><br> | <font size="3"><b>Extraction of p241i and cloning</b></font><br> | ||
<p align="justify"> | <p align="justify"> | ||
| - | 500 µl Eppendorf tubes were pierced at the buttom and sterilized. The filter optained from DNA2.0 was stuffed into the 500 | + | 500 µl Eppendorf tubes were pierced at the buttom and sterilized. The filter optained from DNA2.0 was stuffed into the 500 µl Eppendorf tube that again was put into a 1.5 ml Eppendorf tube. 100 µl MilliQ H<sub>2O</sub> was added and the filter was soaked for 2 minutes. The Eppendorf tubes were centrifuged at 13.000 g for 2 minutes. The eluation was used to <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890372"> chemically transforme </a> <i>E. coli.</i><br> |
| - | Ten random colonies were picked for overnight growth in liquid media. The next day the plasmids were <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890369"> purified</a> and <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890364"> digestion</a> | + | Ten random colonies were picked for overnight growth in liquid media. The next day the plasmids were <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890369"> purified</a> and <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890364"> digestion</a> was performed with SnaBI, BsaI & BamHI to make sure that this was the plasmid we had ordered. The gel showed that this was the case.<br><br> |
<font size="3"><b>Creation of the Leaky construct</b></font><br> | <font size="3"><b>Creation of the Leaky construct</b></font><br> | ||
As desided by our <a href="https://2009.igem.org/Team:DTU_Denmark/genetic_design">genetic design </a> | As desided by our <a href="https://2009.igem.org/Team:DTU_Denmark/genetic_design">genetic design </a> | ||
| - | the redoxilator can be removed from p241i by NotI <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890364"> digestion</a> and subsequent <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890370"> gel-purification</a> of the backbone. Following religation of the backbone the DNA was transformed into E coli. This plasmid was <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890369"> purified</a> and digested with XmaI to check whether the 2500bp & 4500bp appeared. The gel | + | the redoxilator can be removed from p241i by NotI <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890364"> digestion</a> and subsequent <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890370"> gel-purification</a> of the backbone. Following religation of the backbone the DNA was transformed into E coli. This plasmid was <a href="https://2009.igem.org/Team:DTU_Denmark/protocols#_Toc243890369"> purified</a> and digested with XmaI to check whether the 2500bp & 4500bp appeared. The gel gave ambiguous results so a test PCR was done on the purified plasmid to check whether the redoxilator had been removed. This test showed that this was the case.<br><br> |
<font size="3"><b>Transformation into yeast</b></font><br> | <font size="3"><b>Transformation into yeast</b></font><br> | ||
Revision as of 21:12, 21 October 2009

| Home | The Team | The Project | Parts submitted | Modelling | Notebook |
|
The redoxilator - Genetic design - Applications and perspectives - Results - Safety considerations The USER assembly standard - USER fusion of biobricks USER fusion primer design software - Abstract - Instructions - Output format |
The project Results Construction of biobrick BBa_K194003 and yeast plasmid containing biobrick BBa_K194003 for demonstration. Two complentary oligoes were ordered so that they could anneal forming the biobrick BBa_K194003 holding a PacI restriction site flanked by two Nt.BbvCI-sites and with XbaI and SpeI sites in the end for upholding the biobrick standard. TCTAGAGGCTGAGGGTTTAATTAAGACCTCAGCGCAGTGGTGCGATCGCGACACTGCTACTAGT AGATCTCCGACTCCCAAATTAATTCTGGAGTCGCGTCACCACGCTAGCGCTGTGACGATGATCA The two primers were warmed up to 95 °C on PCR machine and slowly cooled down with 5°C every 5 minutes until it reached 25°C for the two primers to anneal correctly. Then the double stranded oligo was digested with XbaI and SpeI o/n (over night). In the morning the oligo was warmed up to 95 °C again and cooled down as former described. This method was chosen to denature the restriction enzyme before ligation as the oligoes would have been lost in a standard DNA purification. The oligo was inserted into two different plasmids: 1. pRS416 : in-house yeast plasmid with URA marker that we wished to use to demonstrate that Biobrick BBa_K194002 worked in yeast. The two plasmids were digested seperately using two enzymes, XbaI (20U) and SpeI (10U). The mixtures were incubated at 37 °C for eight hours after which more enzyme was added to ensure maximum digestion. After an additional two hours of incubation the digested plasmids were dephosphorilised to prevent reanealing during ligation. This was achieved by an Antartic Phosphatase treatment (25 U). The DNA was gel-purified and the resulting concentration was determined using fluorometrics (The Qubit Quantitation PlatformTM from invitrogen). The ligation was performed using T4-DNA ligase using a 3:1 ratio of fragment to vector and 9 ng DNA in total at a volume of 20 µl according to the manufactors recommendations. The actual ligation was carried out on a PCR machine for 1½ hours shifting the temperature from 10 °C to 30 °C at 30 second intervals as desribed by [1]. The final product was finally chemically transformed into competent E coli cells. Selection of transformants Ten random colonies were picked for overnight growth in liquid media. The next day the plasmids were purified and digestion were performed with PacI and XbaI to verify the presence of the biobrick. This was done as the presence of the biobrick is introduced a PacI site in the vector. The XbaI digestion was done to ensure that the biobrick had been inserted with the right directionality.
Figure 1. Left: PacI restriction assay, BBa_K194003 insertion into pRS416. Restriction performed on ten randomly picked, o/n grown and plasmid purified samples. Samples are run on a 1 % agarose gel. Digestion should leave a single band at approxiamately 6 kb. Colonies 5 and 9 are believed to contain the correct plasmid. Right: The ladder used in the corresponding experiment, Hypper Ladder 1. From the gel it was seen that the BBa_K194003 had been inserted correctly into pRS416. Now this was used to demonstrate that biobrick BBa_K194002 was functional. This was done by opening the USER cassette in BBa_K194003 with PacI and Nt.BbvCI, purifying the backbone from gel and adding two PCR fragments: 1. The constitutive TEF promoter (400bp) using the primers BGHA200/370
Figure 2. The cloning strategy for demonstrating that biobricks BBa_K194002 works. Two PCR products were fused using USER fusion and inserted into the pRS416 backbone by USER cloning. After treatment with USERTM mix, the mixture was transformed into E. coli. VerificationFollowing selection of transformants and overnight liquid cultures the plasmids was purified . The plasmids were linearized using AsiSI, which is also included in the BBa_K194003. Plasmids holding the fusion of TEF and BBa_K194002 will show to be 2kb longer than the ones without. The presence of the gene were only verified by PCR of the genes inserted. Extraction of p241i and cloning
500 µl Eppendorf tubes were pierced at the buttom and sterilized. The filter optained from DNA2.0 was stuffed into the 500 µl Eppendorf tube that again was put into a 1.5 ml Eppendorf tube. 100 µl MilliQ H2O was added and the filter was soaked for 2 minutes. The Eppendorf tubes were centrifuged at 13.000 g for 2 minutes. The eluation was used to chemically transforme E. coli. Yeast (CEN PK 113 5D (Ura-)) was transformed with the pRS416c-plasmid using the Ura marker present on pRS416
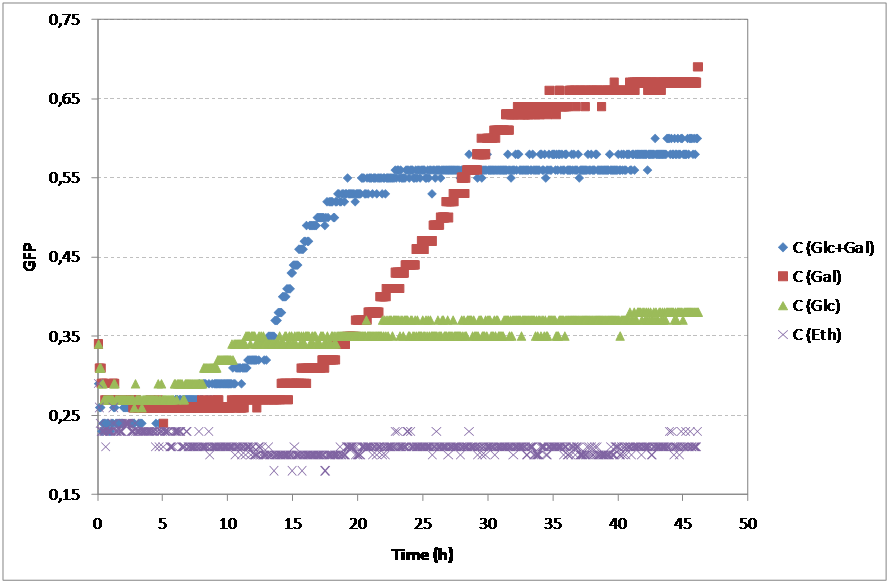
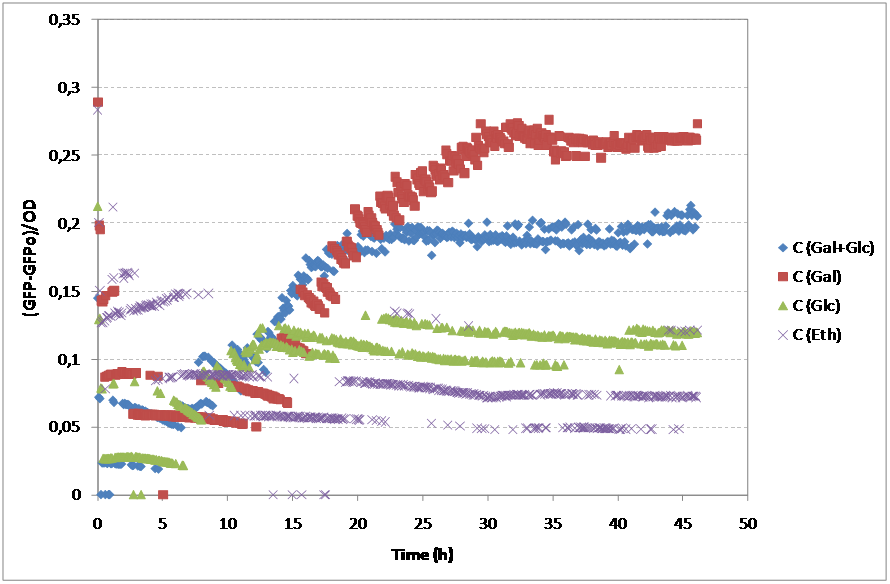
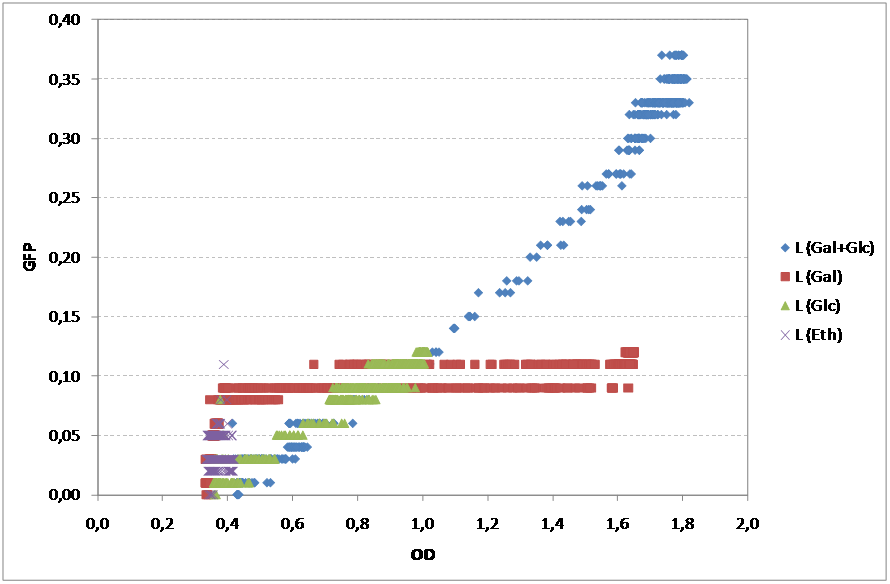
Yeast was also transformed with pSC011 kindly provided by Simon Carlsen, CMB containing the sequence from BBa_K194001 under the constitutive expression of the TEF promoter. pSC011 holds the same promoter and terminator as the pRS416 holding the BBa_K194003 and can therefore be used to analyze the difference of the addition of the Cln2 degradation signal. BioLector fermentation To characterize the yeast strains that we have constructed in the project we used the BioLector machine kindly provided by the Center for Biological Sequence Analysis at DTU. The BioLector is able to keep the microtiterplate stirring and measures OD and GFP levels every 3rd minut making it ideal for our experimentt. Our main goal with this experiment was to determine if there was a difference in GFP signal for different carbon sources and this way prove if the GFP signal is anyway dependent on the NaDH/NAD+ ratio in the cell or not We characterized: 1. Wildtype as background: CEN PK 113 7D without any auxotrophic marker was chosen. If there is GFP fluorescence of the wildtype this needs to be accounted for. 2. Our Redoxilater construct: This construct holds the redoxilator sensor controlling the expression of BBa_K194002. This plasmid was transformed into CEN PK 113 5D (Ura-). 3. Leaky construct: To check whether the redoxilator actually induces expression of BBa_K194002 or our minimal promoter is simply leaky. This plasmid was transformed into CEN PK 113 5D (Ura-). 4. pSC011: Demonstration of BBa_K194001. This plasmid was transformed into CEN PK 113 5D (Ura-). 5. pRS416c: Demonstration of BBa_K194002. This plasmid was transformed into CEN PK 113 5D (Ura-). To minimize the background fluorescence from the media, synthetic complete without riboflavin and folic acid was used since they have been reported to be fluorescent [2]. Growth rates (h-1) obtained for the two strains on diferent carbon sources
As expected, both strains showed different profiles of growth accordingly to the substrate. For Glucose and Galactose, the growth curve showed two exponential phases, the first one corresponding to the growth on Glucose and the second corresponding to the growth on Galactose. Although the initial OD was similar in both cases, the growth on Galactose showed an even longer lag phase than the glucose. Finally, as expected, the substrate reporting less growth was ethanol. For the Redoxilator, the growth solely on Glucose showed a longer lag phase compared to the mixed substrate, probably due to a lower initial OD.
Regarding the GFP signal, all substrates showed different profiles. Nevertheless, the fermentative substrates, Glucose and Galactose, showed higher values of GFP than the respirative substrate, Ethanol.
Although these results are accordingly to what was expected, it is important to mention that these cultures didn’t have comparable growths, so, in order to subtract this variability of the growth, one should normalize the GFP expression with OD. Additionally, to the GFP values, the residual values for each case were subtracted.
[1] Lund, A. H., M. Duch, and F. S. Pedersen. 1996. Increased cloning efficiency by temperature-cycle ligation. Nucleic Acids Res. 24:800-801. doi:l50400 [2] Mark A. Sheff and Kurt S. Thorn. 2004. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 24:800-801. doi:10.1002/yea.1130 |
Achievements
Redox sensing device |
| Comments or questions to the team? Please Email us -- Comments of questions to webmaster? Please Email us |
 "
"