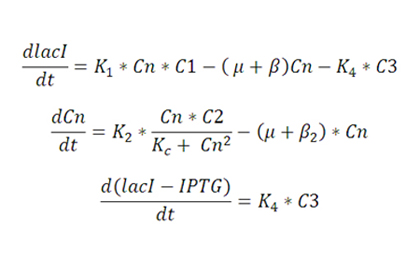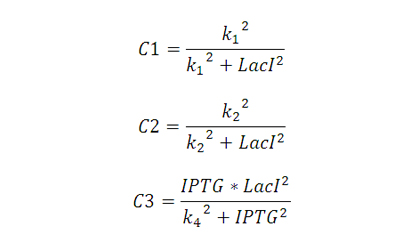Team:IIT Bombay India/PSM
From 2009.igem.org
Pranayiitb (Talk | contribs) |
Pranayiitb (Talk | contribs) |
||
| (10 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
!align="left"| | !align="left"| | ||
| - | | | + | | |
| - | + | ||
Stochastic Modelling for the system | Stochastic Modelling for the system | ||
| - | Objective | + | |
| - | + | ||
| - | Model | + | '''Objective''' |
| + | To characterize the intrinsic noise present in the system for all the 4 strains. | ||
| + | To compare the lac I expression levels and plasmid concentrations and the errors associated with them for each of the 4 strains, using a simplified phenomenological model. | ||
| + | |||
| + | '''Model''' | ||
| + | |||
A simplified model for lacI expression and copy number regulation is developed. | A simplified model for lacI expression and copy number regulation is developed. | ||
| - | |||
| - | |||
| - | |||
| + | [[Image:Eq-1.jpg]] | ||
| + | |||
| + | Where the terms C1 and C2 and C3 representing control action are: | ||
| + | |||
| + | [[Image:Eq-2.jpg]] | ||
| + | |||
| + | For open loop, none of the control actions exist, and hence C1=C2=1 and C3=0; | ||
| + | |||
| + | For the strain with lacI regulation, C2=1, C1 and C3 are obtained from the equations above. | ||
| + | |||
| + | For strain with plasmid regulation, C1=1,C2 and C3 are obtained from the equations above. | ||
| + | |||
| + | For strain with multiple feedback, all the three terms, C1, C2 and C3 are obtained from equations above. | ||
| + | |||
| + | |||
| + | '''Methodology''' | ||
| + | |||
| + | Stochasticity is introduced in the system by randomly perturbing the kinetic parameters, K1,K2, K3 and K4 and k1, k2 and k4 from their mean values to a maximum limit of 30 % and carrying out numerous simulations to obtain the various trajectories possible. Hence the distributions so obtained for lacI and plasmid concentrations are characterized . The errors in these distributions are then compared for the 4 strains. | ||
| + | |||
| + | |||
| + | '''Results''' | ||
| + | |||
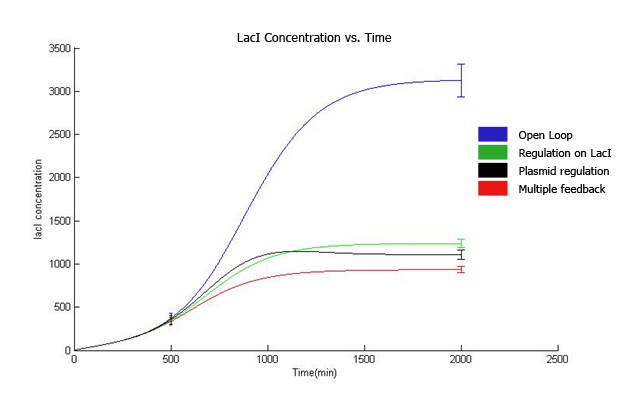
| + | [[Image:Graph-1.jpg]] | ||
| + | |||
| + | The qualitative behavior for all the 4 strains is similar. | ||
| + | |||
| + | The strain with multiple feedback shows least expression while strain with no feedback shows maximum expression. | ||
| + | |||
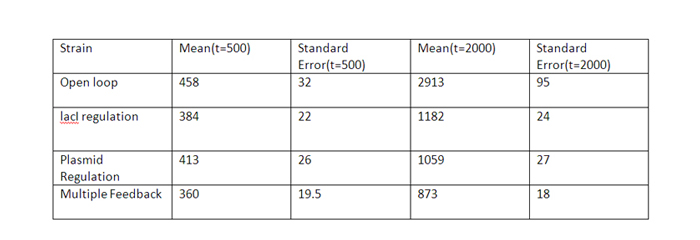
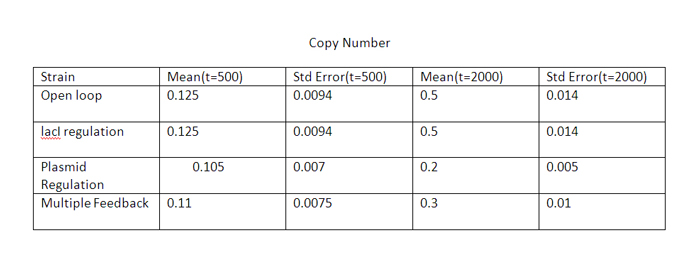
| + | The error bars are plotted above. The mean values for 100 runs and the errors associated with them are summarized below. | ||
| + | |||
| + | [[Image:Table-1.jpg]] | ||
| + | |||
| + | Thus, error decreases almost 6 times for the strain with multiple feedback as compared to the open loop strain. | ||
| + | |||
| + | The error is almost similar for strains with only a single feedback, which is less than that for the open loop strain. | ||
| + | |||
| + | [[Image:Graph-2.jpg]] | ||
| + | |||
| + | [[Image:Table-2.jpg]] | ||
| + | |||
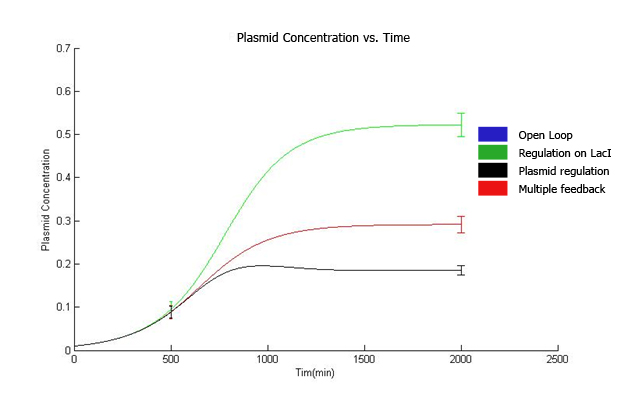
| + | The curves for plasmid concentration in the open loop strain and strain with lacI regulation are the same, since plasmid replication is unaffected by lacI regulation. | ||
| + | |||
| + | The error in plasmid concentration is least in the strain with plasmid regulation, it is 1/3rd of the error in open loop strain. | ||
| + | This can be attributed to the fact that the lacI feeds back to two control loops in strain with multiple feedback, and hence it does not regulate the plasmid concentration as effectively. | ||
| + | |||
| + | '''Effect of IPTG on system:''' | ||
| + | |||
| + | [[Image:Graph-3.jpg]] | ||
| + | |||
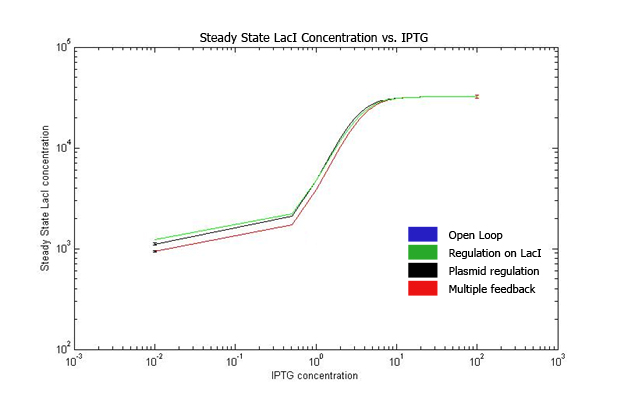
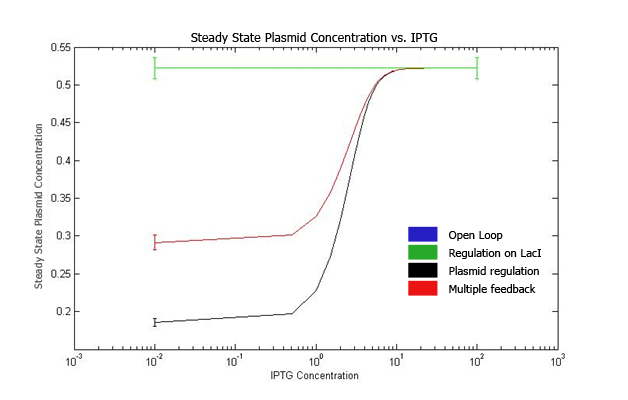
| + | Increasing IPTG causes all systems to resemble open loop in their behavior, which is confirmed by their steady state concentration. | ||
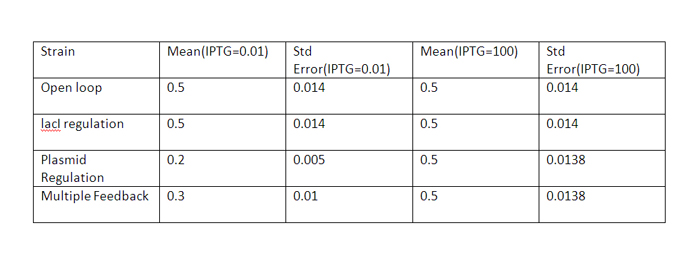
| + | The error values are summarized. | ||
| + | |||
| + | [[Image:Table-3.jpg]] | ||
| + | |||
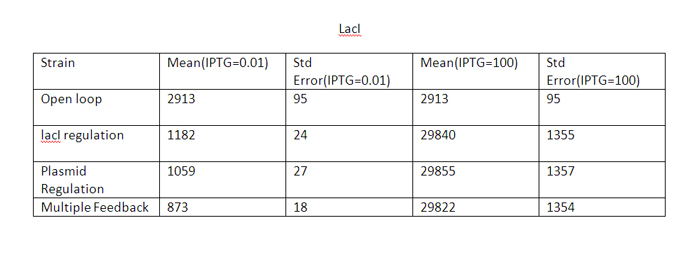
| + | At high IPTG, the error and mean is almost similar for all strains. | ||
| + | Note that we plot the total IPTG present in the system(free as well as complexed with IPTG). Hence the high values observed in the 3 strains. | ||
| + | |||
| + | [[Image:Graph-4.jpg]] | ||
| + | |||
| + | [[Image:Table-4.jpg]] | ||
| + | |||
| + | Here again, the resemblance of the system with open loop is observed at high IPTG values. | ||
| + | |||
| + | This is confirmed by the same mean and errors obtained at high IPTG values. | ||
| + | |||
| + | Thus, we see that the strain with multiple feedback shows greater degree of control with reduced noise. | ||
| + | |||
| + | Further we are attempting to study the differences on growth on lactose in the 4 strains by introducing stochasticity on the reduced model. | ||
| + | |||
| + | The detailed methodology, system equations, results and discussion can be seen [[Media:Stochastic modelling.pdf|here]]. | ||
| - | |||
| - | |||
| - | |||
| | | | ||
|} | |} | ||
Latest revision as of 01:02, 22 October 2009
| Home | The Team | The Project | Analysis | Modeling | Notebook | Safety |
|---|
Phenomenological Stochastic Model |
|
Stochastic Modelling for the system
Model A simplified model for lacI expression and copy number regulation is developed. Where the terms C1 and C2 and C3 representing control action are: For open loop, none of the control actions exist, and hence C1=C2=1 and C3=0; For the strain with lacI regulation, C2=1, C1 and C3 are obtained from the equations above. For strain with plasmid regulation, C1=1,C2 and C3 are obtained from the equations above. For strain with multiple feedback, all the three terms, C1, C2 and C3 are obtained from equations above.
Stochasticity is introduced in the system by randomly perturbing the kinetic parameters, K1,K2, K3 and K4 and k1, k2 and k4 from their mean values to a maximum limit of 30 % and carrying out numerous simulations to obtain the various trajectories possible. Hence the distributions so obtained for lacI and plasmid concentrations are characterized . The errors in these distributions are then compared for the 4 strains.
The qualitative behavior for all the 4 strains is similar. The strain with multiple feedback shows least expression while strain with no feedback shows maximum expression. The error bars are plotted above. The mean values for 100 runs and the errors associated with them are summarized below. Thus, error decreases almost 6 times for the strain with multiple feedback as compared to the open loop strain. The error is almost similar for strains with only a single feedback, which is less than that for the open loop strain. The curves for plasmid concentration in the open loop strain and strain with lacI regulation are the same, since plasmid replication is unaffected by lacI regulation. The error in plasmid concentration is least in the strain with plasmid regulation, it is 1/3rd of the error in open loop strain. This can be attributed to the fact that the lacI feeds back to two control loops in strain with multiple feedback, and hence it does not regulate the plasmid concentration as effectively. Effect of IPTG on system: Increasing IPTG causes all systems to resemble open loop in their behavior, which is confirmed by their steady state concentration. The error values are summarized. At high IPTG, the error and mean is almost similar for all strains. Note that we plot the total IPTG present in the system(free as well as complexed with IPTG). Hence the high values observed in the 3 strains. Here again, the resemblance of the system with open loop is observed at high IPTG values. This is confirmed by the same mean and errors obtained at high IPTG values. Thus, we see that the strain with multiple feedback shows greater degree of control with reduced noise. Further we are attempting to study the differences on growth on lactose in the 4 strains by introducing stochasticity on the reduced model. The detailed methodology, system equations, results and discussion can be seen here.
|
 "
"