Team:DTU Denmark/theory
From 2009.igem.org
| Line 232: | Line 232: | ||
<html> | <html> | ||
| - | <p align="justify"><i><b>Figure 2 – Schematic overview of overall approach.</b><br> | + | <p align="justify"><i><b>Figure 2 – Schematic overview of overall approach.</i></b><br> |
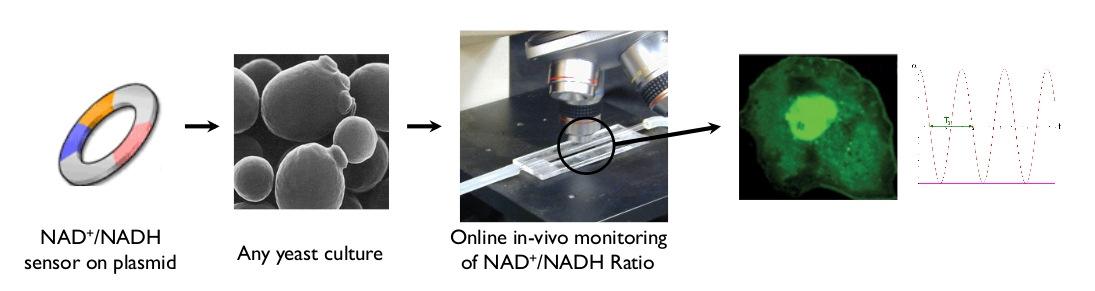
After the design, synthesis and transformation of the NAD<sup>+</sup>/NADH sensor, online measurement of reporter gene expression will be measured and oscillative behaviour of the productivity will be evaluated and used for further optimization.<br><br> | After the design, synthesis and transformation of the NAD<sup>+</sup>/NADH sensor, online measurement of reporter gene expression will be measured and oscillative behaviour of the productivity will be evaluated and used for further optimization.<br><br> | ||
The measuring of cellular NAD<sup>+</sup>/NADH levels is usually a difficult process, especially due to fast changes in NAD<sup>+</sup>/NADH ratio that can occur when a sample is taken and exposed to slightly new conditions. With this sensor system, the quantitative measurement of the reporter gene will provide a fast and reliable way to determine the NAD<sup>+</sup>/NADH ratio in vivo. The plasmid can be transformed into a yeast strain allowing the NAD<sup>+</sup>/NADH ratio to be continuously monitored. As an example this plasmid can be used to study whether an engineered yeast strain has an altered NAD<sup>+</sup>/NADH ratio. The plasmid can be transformed into different yeast strains (e.g. wild type versus an engineered strain or two production strains) and the NAD<sup>+</sup>/NADH ratio can be compared.<br><br> | The measuring of cellular NAD<sup>+</sup>/NADH levels is usually a difficult process, especially due to fast changes in NAD<sup>+</sup>/NADH ratio that can occur when a sample is taken and exposed to slightly new conditions. With this sensor system, the quantitative measurement of the reporter gene will provide a fast and reliable way to determine the NAD<sup>+</sup>/NADH ratio in vivo. The plasmid can be transformed into a yeast strain allowing the NAD<sup>+</sup>/NADH ratio to be continuously monitored. As an example this plasmid can be used to study whether an engineered yeast strain has an altered NAD<sup>+</sup>/NADH ratio. The plasmid can be transformed into different yeast strains (e.g. wild type versus an engineered strain or two production strains) and the NAD<sup>+</sup>/NADH ratio can be compared.<br><br> | ||
| + | |||
| + | <b><i>ii) Product formation regulated by the Rexivator – an attempt to improve and prolong chemostat processes</i></b><br> | ||
| + | When S. cerevisiae are grown continuously in a chemostat, the productivity of e.g. antibiotic or protein product | ||
| + | gradually decreases [Personal correspondence with Novo Nordisk A/S]. This occurs during maximum production and is believed to be the result of metabolic adaption8 with reduced product formation as a consequence. The metabolic adaption is believed to occur because the cells are stressed by the extensive production. The cells will adapt to the new metabolic situation, which will gradually lead to lower production rates. This is highly undesirable in the biotech industry, as the chemostats will have to be restarted on a regular basis, which is costly and time consuming.<br><br> | ||
Revision as of 19:39, 8 October 2009

| Home | The Team | The Project | Parts submitted | Modelling | Notebook |
|
The redoxilator - Theoretical background - Yeast as a model organism - Practical approach The USER assembly standard - Principle - Proof of concept - Manual - Primer design software |
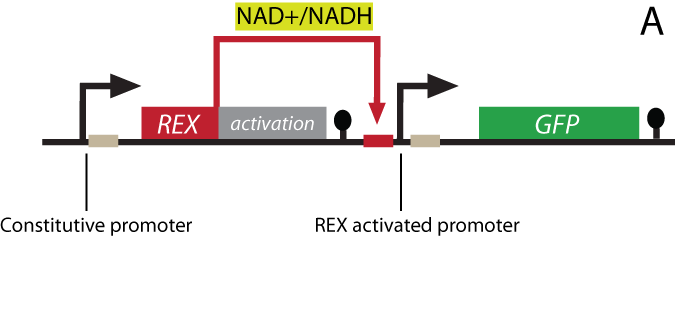
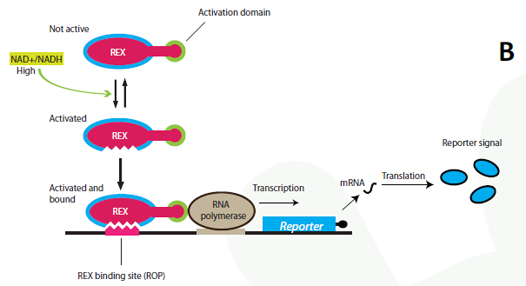
The project Theoretical background The NAD+/NADH ratio sensor-protein Rex (Redox regulator) has been discovered in the bacterium Streptomyces coelicolor. In its host organism, the sensor works as a repressor and controls the gene expression of a large number of genes by recognizing and binding to a specific DNA-sequence termed ROP (Rex OPerator). NAD+ and NADH compete for Rex binding, and the protein binds the ROP DNA-sequence only when NAD+ is bound. Our synthetic biology project: The Redoxilator To achieve a system that senses changing levels in the NAD+/NADH ratio in the eukaryote S. cerevisiae, the gene encoding the Rex protein will be fused to a yeast activator domain, resulting in a new synthetic protein: the Redoxilator. The ROP sequence - the DNA binding site Rex can bind to - will be inserted into a yeast promoter, resulting in a promoter activated by the Redoxilator.
Figure 1 - Gene design and redox regulation
A certain NAD+/NADH ratio will activate the Redoxilator to recognize the ROB promoter resulting in transcription of a downstream gene. In this way the ROB promoter and the Redoxilator comprises the complete sensing system. The system can be coupled to the expression of virtually any gene of interest; making transcription solely dependent on the ratio of NAD+/NADH in the cell. In our iGEM project, the system will be used for two selected applications considered highly relevant: i) in vivo monitoring of NAD+/NADH in yeast, and ii) NAD+/NADH ratio regulated production of yeast products in chemostat processes. i) Reporter gene expression regulated by the Rexivator – an in vivo redox sensorThe gene encoding green fluorescent protein (GFP) is widely used as a reporter gene in molecular biology. By placing the ROB promoter upstream of a GFP gene on a plasmid, and transforming the whole system into a yeast cell, GFP will be expressed at certain NAD+/NADH levels. When the Rexivator is bound to DNA, GFP expression will produce a visible and quantitatively measurable signal, which will be an indirect measure of the NAD+/NADH ratio.
Figure 2 – Schematic overview of overall approach. |
The yeast metabolic cycle It has recently been shown by Tu et al. and Klevecz et al. that the expression of at least half of the genes monitored on a standard yeast gene chip will oscillate in a coordinated manner when grown under glucose limited conditions. The cells will shift between oxidative and reductive metabolism in a synchronized metabolic cycle with three phases: oxidative, reductive/building and reductive/ charging. As oxygen will only be consumed in the oxidative phase, the dissolved oxygen will oscillate. Many metabolites and cofactors including NADH and NAD+ will also oscillate during this cycle as NADH is converted to NAD+ when oxygen is consumed. |
| Comments or questions to the team? Please Email us -- Comments of questions to webmaster? Please Email us |
 "
"