Team:Heidelberg/Project Highlights
From 2009.igem.org
m (→4 RFCs, well characterized parts) |
HannahMeyer (Talk | contribs) (→4 RFCs, well characterized parts) |
||
| (20 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
__NOTOC__ | __NOTOC__ | ||
= Project Highlights = | = Project Highlights = | ||
| + | |||
| + | * [[Team:Heidelberg/Project_Highlights#We_are_able_to_predict_functional_mammalian_promoter_sequences|We are able to predict functional mammalian promoter sequences]] | ||
| + | * [[Team:Heidelberg/Project_Highlights#We_have_created_a_functional_biochemical_synthesis_method_for_the_generation_of_promoters_libraries|We have created a functional biochemical synthesis method for the generation of promoters libraries]] | ||
| + | * [[Team:Heidelberg/Project_Highlights#We_have_developed_novel_standards_for_measurement_of_promoters_in_mammalian_cells|We have developed novel standards for measurement of promoters in mammalian cells]] | ||
| + | * [[Team:Heidelberg/Project_Highlights#4_RFCs,_well_characterized_parts|4 RFCs, well characterized parts]] | ||
== We are able to predict functional mammalian promoter sequences == | == We are able to predict functional mammalian promoter sequences == | ||
| - | <span style="font-size:4mm">We created [[Team:Heidelberg/HEARTBEAT|HEARTBEAT]], a model portfolio based upon genome-wide bioinformatic analysis and ''fuzzy logic''. We used these tools to predict promoter sequences | + | <span style="font-size:4mm">We created [[Team:Heidelberg/HEARTBEAT|HEARTBEAT]], a model portfolio based upon genome-wide bioinformatic analysis and ''fuzzy logic''. We used these tools to predict promoter sequences and subsequently synthesized those sequences. We found that promoters most closely matching the model predictions work, whereas others do not (Fig 1). Read more about these results on the [[Team:Heidelberg/HEARTBEAT_database| "HEARTBEAT database"]] page. We also present a [[Team:Heidelberg/HEARTBEAT_GUI|GUI]] which enables the users to design the promoter of their needs.</span> |
| - | [[Image:HD09_highlight.jpg|thumb|none|480px|HEARTBEAT describes the spatial distribution of Transcription Factor Binding Sites along a mammalian promoter. The diagram on the top shows this distribution for [[Team:Heidelberg/Eukaryopedia#SREBP|SREBP]]. HEARTBEAT also describes Transcription Factors co-occurring with a given TF, which, for SREBP, is [[Team:Heidelberg/Eukaryopedia#Sp1|Sp1]]. We placed SREBP and Sp1 Binding sites according to the predicted distribution and found only promoters which resemble the distribution closest to be induced by SREBP (see small diagram). Other promoter sequences are not functional]] | + | {| |
| + | |[[Image:HD09_highlight.jpg|thumb|none|480px|<div style="text-align:justify;">'''Fig 1: HEARTBEAT describes the spatial distribution of Transcription Factor Binding Sites along a mammalian promoter'''. The diagram on the top shows this distribution for [[Team:Heidelberg/Eukaryopedia#SREBP|SREBP]]. HEARTBEAT also describes Transcription Factors co-occurring with a given TF, which, for SREBP, is [[Team:Heidelberg/Eukaryopedia#Sp1|Sp1]]. We placed SREBP and Sp1 Binding sites according to the predicted distribution and found only promoters which resemble the distribution closest to be induced by SREBP (see small diagram). Other promoter sequences are not functional (not shown).]]</div> | ||
| + | |} | ||
== We have created a functional biochemical synthesis method for the generation of promoters libraries == | == We have created a functional biochemical synthesis method for the generation of promoters libraries == | ||
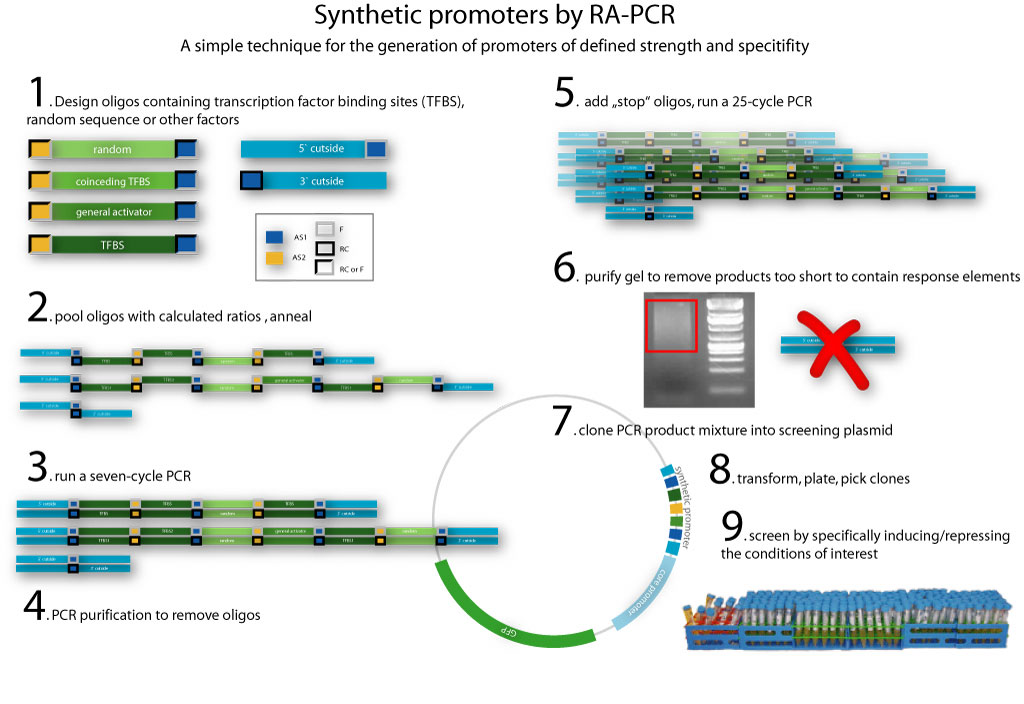
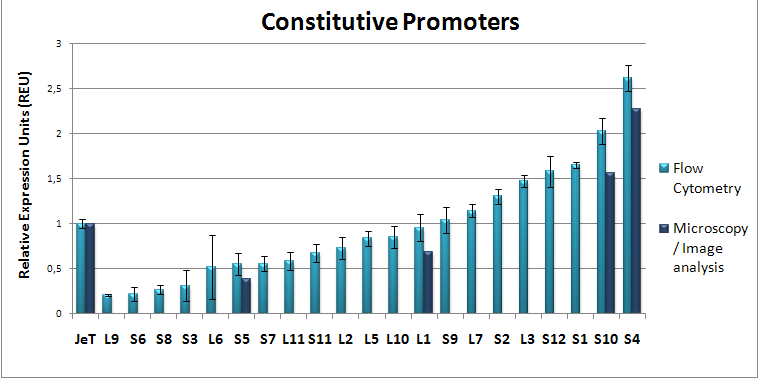
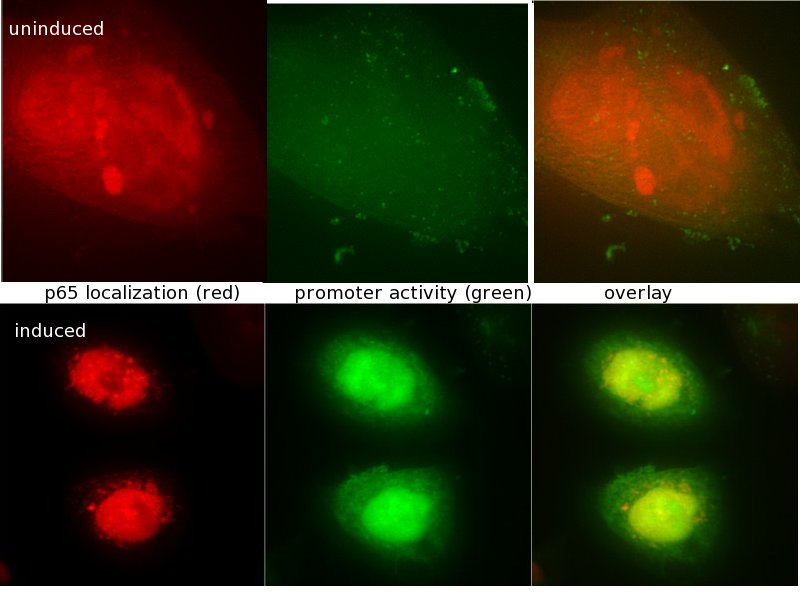
| - | <span style="font-size:4mm">We developed [[Team:Heidelberg/Project_Synthetic_promoters|RA-PCR]], a biochemical method for the generation of randomized promoter libraries. | + | <span style="font-size:4mm">We developed [[Team:Heidelberg/Project_Synthetic_promoters|RA-PCR]], a biochemical method for the generation of randomized promoter libraries (Fig. 2). Using this method we created a library of constitutive promoters (Fig. 3), an NF-κB responsive promoter which was subjected to extensive characterization (Fig. 4) as well as some other regulated promoters .</span> |
| - | [[Image:HD09_rapcr.jpg|none|thumb|750px|<div style="text-align:justify;">The method of RA-PCR. | + | [[Image:HD09_rapcr.jpg|none|thumb|750px|<div style="text-align:justify;">'''Fig. 2: The method of RA-PCR'''. Oligos and transcription factor binding sites (TFBS) and mutually annealing sequences are combined in a single PCR reaction. Randomized repeats of such TFBSs were generated at random spacing, making it likely to produce functional promoters at different strength.</div>]] |
{| class="wikitable centered" border="0" rules="rows" style="border-color:white;" | {| class="wikitable centered" border="0" rules="rows" style="border-color:white;" | ||
|- | |- | ||
| - | |[[Image:HD09_constitutive.png|thumb|none|350px|<div style="text-align:justify;">A library of constitutive promoters of different strength created by RA-PCR.</div>]] | + | |[[Image:HD09_constitutive.png|thumb|none|350px|<div style="text-align:justify;">'''Fig. 3: A library of constitutive promoters of different strength''' created by RA-PCR.</div>]] |
| - | |[[Image:HD09_nfkappa_onoff.jpg|thumb|none|350px|<div style="text-align:justify;">NF-κB regulated promoter created by RA-PCR which | + | |[[Image:HD09_nfkappa_onoff.jpg|thumb|none|350px|<div style="text-align:justify;">'''Fig. 4: NF-κB regulated promoter created by RA-PCR.'''It is activated, which is detected by the green fluorescence of the reporterprotein if NF-κB localizes to the nucleus detected by p65-mCherry fusion protein (red fluorescence within the nucleus)</div>]] |
|} | |} | ||
== We have developed novel standards for measurement of promoters in mammalian cells == | == We have developed novel standards for measurement of promoters in mammalian cells == | ||
| - | <span style="font-size:4mm">Applying both qRT-PCR and flow cytometry, we developed two new relative units in analogy to bacterial RPU for use in mammalians: Relative Expression Units and Relative Mammalian Promoter Units. We | + | <span style="font-size:4mm">Applying both qRT-PCR and flow cytometry, we developed two new relative units in analogy to bacterial RPU for use in mammalians: Relative Expression Units (Fig. 5) and Relative Mammalian Promoter Units (Fig. 6). We applied these units to the characterization of our promoters, as well as an [http://partsregistry.org/Part:BBa_I712004 existing promoter from the registry]. Read more about our[[Team:Heidelberg/Project_Measurement| Measurement subproject]]...</span> |
{| | {| | ||
|-valign="top" border="0" | |-valign="top" border="0" | ||
| - | |width=" | + | |width="350px" style="padding: 0 20px 0 0;"| |
| - | [[Image:HD09_CMV_standard3.png|center| | + | [[Image:HD09_CMV_standard3.png|center|350px|thumb|<div style="text-align:justify;">'''Fig. 5 :Strength of the CMV promoter in different cell lines in REU'''. [https://2009.igem.org/Team:Heidelberg/Project_Measurement Relative Expression Units (REU)] were determined by three independent flow cytometry measurements on three different days. This measurement was confirmed by an independent technique - image analysis.</div>]] |
| - | |width=" | + | |width="350px" style="padding: 0 20px 0 0;"| |
| - | [[Image:Qpcr_result.png|center| | + | [[Image:Qpcr_result.png|center|350px|thumb|<div style="text-align:justify;">'''Fig. 6: Real-time RT-PCR data of CMV promoter'''. Twelve replicates of HeLa cells were transfected with a plasmid containing the CMV promoter coupled to GFP. Another set of twelve replicates was transfected with the JeT promoter coupled to GFP served as reference. Total amount of RNA generated by CMV was divided by the total amount of RNA generated by JeT to obtain [https://2009.igem.org/Team:Heidelberg/Project_Measurement RMPU]. </div>]] |
|- | |- | ||
|} | |} | ||
| Line 41: | Line 48: | ||
== 4 RFCs, well characterized parts == | == 4 RFCs, well characterized parts == | ||
| - | <span style="font-size:4mm"> | + | <span style="font-size:4mm">According to the standards required by synthetic biology we submitted all the methods and units we developed as RFCs (Request for Comments). By introducing new standards for synthetic biology in mammalian cells, we hope to initiate a process that will allow the number of mammalian parts in the registry to skyrocket within the next years:</span> |
* <span style="font-size:4mm">[http://hdl.handle.net/1721.1/49501 RCF 41]: Units for Promoter Measurement in Mammalian Cells</span> | * <span style="font-size:4mm">[http://hdl.handle.net/1721.1/49501 RCF 41]: Units for Promoter Measurement in Mammalian Cells</span> | ||
* <span style="font-size:4mm">[http://hdl.handle.net/1721.1/49502 RFC 42]: RA-PCR, a method for the generation of randomized promoter libraries</span> | * <span style="font-size:4mm">[http://hdl.handle.net/1721.1/49502 RFC 42]: RA-PCR, a method for the generation of randomized promoter libraries</span> | ||
Latest revision as of 18:49, 21 October 2009
Project Highlights
We are able to predict functional mammalian promoter sequencesWe created HEARTBEAT, a model portfolio based upon genome-wide bioinformatic analysis and fuzzy logic. We used these tools to predict promoter sequences and subsequently synthesized those sequences. We found that promoters most closely matching the model predictions work, whereas others do not (Fig 1). Read more about these results on the "HEARTBEAT database" page. We also present a GUI which enables the users to design the promoter of their needs.
We have created a functional biochemical synthesis method for the generation of promoters librariesWe developed RA-PCR, a biochemical method for the generation of randomized promoter libraries (Fig. 2). Using this method we created a library of constitutive promoters (Fig. 3), an NF-κB responsive promoter which was subjected to extensive characterization (Fig. 4) as well as some other regulated promoters . We have developed novel standards for measurement of promoters in mammalian cellsApplying both qRT-PCR and flow cytometry, we developed two new relative units in analogy to bacterial RPU for use in mammalians: Relative Expression Units (Fig. 5) and Relative Mammalian Promoter Units (Fig. 6). We applied these units to the characterization of our promoters, as well as an [http://partsregistry.org/Part:BBa_I712004 existing promoter from the registry]. Read more about our Measurement subproject...
4 RFCs, well characterized partsAccording to the standards required by synthetic biology we submitted all the methods and units we developed as RFCs (Request for Comments). By introducing new standards for synthetic biology in mammalian cells, we hope to initiate a process that will allow the number of mammalian parts in the registry to skyrocket within the next years:
We also submit more than 10 well-characterized parts to the registry (see Parts Characterization). Also, we submit several other parts, for which we offer at least a rough characterization (Find our full parts list here)
|
 "
"