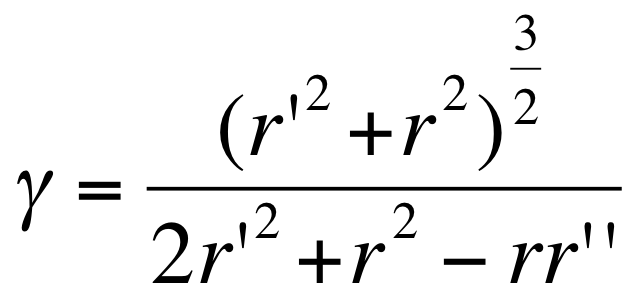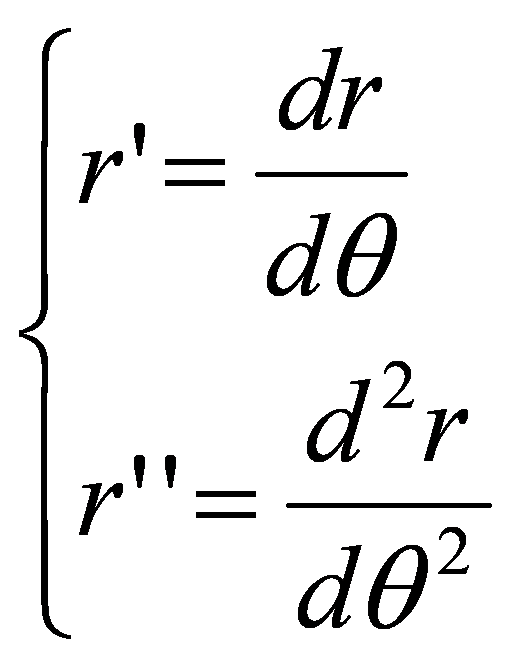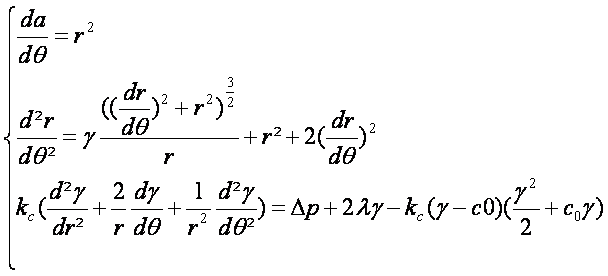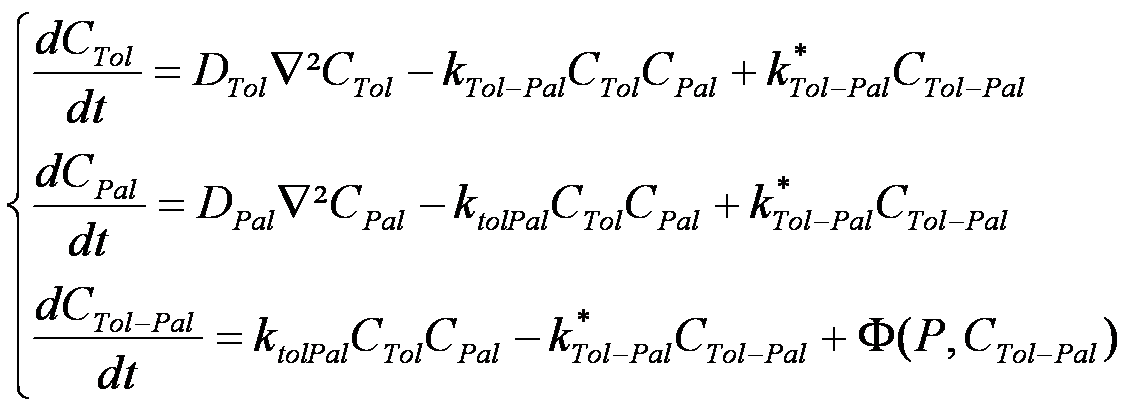Team:Paris/Production modeling2
From 2009.igem.org
Gregory Batt (Talk | contribs) (→Tol/Pal accumulates at the basis of nascent blebs) |
|||
| (63 intermediate revisions not shown) | |||
| Line 54: | Line 54: | ||
width: 30px; | width: 30px; | ||
top: 35px; | top: 35px; | ||
| - | left: | + | left: 10px; |
margin-top:10px; | margin-top:10px; | ||
padding-top: 7px; | padding-top: 7px; | ||
| - | background: url(https://static.igem.org/mediawiki/2009/ | + | background: url(https://static.igem.org/mediawiki/2009/4/40/Left_menu_paris2.png); |
z-index:4; | z-index:4; | ||
} | } | ||
| Line 63: | Line 63: | ||
#middle-side2 { | #middle-side2 { | ||
height: 25px; | height: 25px; | ||
| - | width: | + | width: 650px; |
position: absolute; | position: absolute; | ||
top: 35px; | top: 35px; | ||
| - | left: | + | left: 20px; |
margin-top:10px; | margin-top:10px; | ||
padding-top: 5px; | padding-top: 5px; | ||
| Line 80: | Line 80: | ||
padding-top: 7px; | padding-top: 7px; | ||
top: 35px; | top: 35px; | ||
| - | left: | + | left: 650px; |
| - | background: url(https://static.igem.org/mediawiki/2009/ | + | background: url(https://static.igem.org/mediawiki/2009/f/f8/Right_menu_paris2.png); |
z-index:4; | z-index:4; | ||
} | } | ||
| Line 95: | Line 95: | ||
padding-left: 7px; | padding-left: 7px; | ||
padding-right: 7px; | padding-right: 7px; | ||
| - | color: | + | color:#b0310e; |
font-weight:bold; | font-weight:bold; | ||
} | } | ||
| Line 110: | Line 110: | ||
<div id="left-side2"></div> | <div id="left-side2"></div> | ||
<div id="middle-side2"><center> | <div id="middle-side2"><center> | ||
| - | <a class="menu_sub_active"href="https://2009.igem.org/Team:Paris/ | + | <a class="menu_sub_active"href="https://2009.igem.org/Team:Paris/Production_modeling2#bottom">Intro</a>| |
| - | <a class=" | + | <a class="menu_sub_active"href="https://2009.igem.org/Team:Paris/Production_modeling2#2">Membrane model</a>| |
| - | <a class=" | + | <a class="menu_sub_active"href="https://2009.igem.org/Team:Paris/Production_modeling2#3">Tol/Pal model</a>| |
| - | <a class=" | + | <a class="menu_sub_active"href="https://2009.igem.org/Team:Paris/Production_modeling2#4">Bleb initiation</a>| |
| - | <a class=" | + | <a class="menu_sub_active"href="https://2009.igem.org/Team:Paris/Production_modeling2#5">Tol/Pal accumulation</a>| |
| + | <a class="menu_sub_active"href="https://2009.igem.org/Team:Paris/Production_modeling2#6">Vesiculation</a> | ||
</center> | </center> | ||
</div> | </div> | ||
| Line 120: | Line 121: | ||
</html> | </html> | ||
| - | + | <span/ id="1"> | |
| - | + | ||
This part aims at investigating an important issue regarding the creation of vesicles: | This part aims at investigating an important issue regarding the creation of vesicles: | ||
| Line 151: | Line 151: | ||
We assumed that the intracellular membrane position is fixed (pushed against the peptidoglycan layer). | We assumed that the intracellular membrane position is fixed (pushed against the peptidoglycan layer). | ||
| + | <span/ id="2"> | ||
| + | ===Modeling membrane at steady state=== | ||
| - | + | The objective of this part is to discuss the existence of an intrinsic curvature of the outer membrane at steady state, and to obtain an estimate of its characteristic parameter. | |
| - | |||
| + | Lipopolysaccharides (LPSs) are important constituents of the ''E. coli'' outer membrane. | ||
| + | They possess long polycaccharide chains pointing outwards. | ||
| + | These extracellular sugar extensions create mutual attraction forces that curb the membrane. | ||
| + | This situation is depicted in the figure below. | ||
| + | |||
| + | |||
| + | [[Image:LPS_CLUSTER_Wiki.png|250px|center]] | ||
| + | |||
| + | |||
| + | From a physical point of view, the lipid bilayer behaves like a liquid at 37°c. | ||
| + | To each membrane conformation, one can associate an energy, called the bending energy [1]. | ||
| + | This is given by : | ||
| + | |||
| + | [[Image:Bending energy.png|220px|center]] | ||
| + | |||
| + | where E is the energy of the membrane, K<sub>b</sub> and K<sub>g</sub> are Bending and Gaussian moduli, H<sub>g</sub> is the Gaussian curvature and c<sub>0</sub> is a parameter describing the intrinsic curvature of the outer membrane at steady state (ie, minimal energy). The shape of the membrane is described locally by the principal curvature variables c<sub>1</sub> and c<sub>2</sub> (see [http://en.wikipedia.org/wiki/Principal_curvature here] for details). dS is an infinitesimal surface element. | ||
| - | |||
| - | |||
| - | |||
| - | + | Under simple assumptions on the shape of ''E. coli'' (seen as a cylinder) and assuming that on average the size of the vesicles is the one requiring a minimum of energy, we can using the above relation relate the radius of ''E. coli'' r, the mean radius of vesicles r', and the γ<sub>0</sub> parameter: | |
| - | + | [[Image:Relations.png|180px|center]] | |
| - | + | Using r= 300 nm and r’=100 nm (the vesicle size ranges from 25 nm to 175 nm), we estimate the outer membrane intrinsic curvature as | |
| + | |||
| - | + | [[Image:gamma0.png|150px|center]] | |
| - | + | Obtaining an estimation of this parameter is essential to model and simulate the membrane dynamic deformation under osmotic pressure differences as we will see. | |
| - | + | ||
| - | + | ||
===Modeling membrane deformation=== | ===Modeling membrane deformation=== | ||
| Line 182: | Line 195: | ||
Because of osmotic pressure differences, we assumed that the intracellular membrane is pushed against the -perfectly circular- peptidoglycan layer. So, only outer membrane deformations have to be modeled. | Because of osmotic pressure differences, we assumed that the intracellular membrane is pushed against the -perfectly circular- peptidoglycan layer. So, only outer membrane deformations have to be modeled. | ||
| - | The difference of osmotic pressure between the intra-cellular environment and the periplasm, and between the periplasm and the extra-cellular environment comes notably from the turnover of peptidoglycan molecules [ | + | The difference of osmotic pressure between the intra-cellular environment and the periplasm, and between the periplasm and the extra-cellular environment comes notably from the turnover of peptidoglycan molecules <sup>[[https://2009.igem.org/Team:Paris/Production_modeling2#References 1]]</sup>. This hypothesis has been made by Zhou & Doyle <sup>[[https://2009.igem.org/Team:Paris/Production_modeling2#References 3]]</sup> |
| + | |||
| + | To model membrane deformation, we use the equation proposed by Ou-Yang and Helfrich <sup>[[https://2009.igem.org/Team:Paris/Production_modeling2#References 4]]</sup>: | ||
| + | |||
| + | [[Image:Diferential System.png|300px|center]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
After simplifications, we obtain the following equality. | After simplifications, we obtain the following equality. | ||
| Line 195: | Line 208: | ||
| - | In this equation, γ is the variable membrane curvature, Δp is the osmotic pression difference, c<sub>0</sub> is the membrane intrinsic curvature, and other parameters are as described in | + | In this equation, γ is the variable membrane curvature, Δp is the osmotic pression difference, c<sub>0</sub> is the membrane intrinsic curvature, and other parameters are as described in Ou-Yang and Helfrich <sup>[[https://2009.igem.org/Team:Paris/Production_modeling2#References 4]]</sup>. |
| Line 223: | Line 236: | ||
In addition, '''we decided to model the role of the Tol/Pal system as boundary condition for the system of differential equations : cluster of Tol-Pal are considered as a point with a radius equal to peptidoglycan's one plus the length of the protein. ''' Furthermore, as the surface is closed, we must impose the fact that r(0)=r(2π) to account for closing the vesicle. | In addition, '''we decided to model the role of the Tol/Pal system as boundary condition for the system of differential equations : cluster of Tol-Pal are considered as a point with a radius equal to peptidoglycan's one plus the length of the protein. ''' Furthermore, as the surface is closed, we must impose the fact that r(0)=r(2π) to account for closing the vesicle. | ||
| + | <span/ id="3"> | ||
| + | ===Modeling Tol/Pal diffusion=== | ||
| - | |||
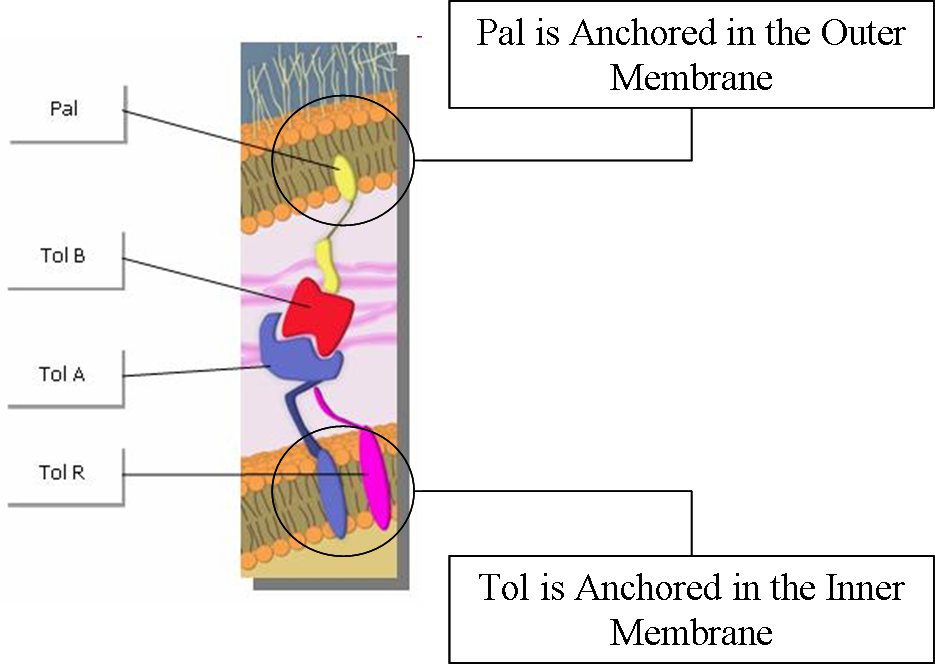
Tol and Pal are membrane proteins which are located respectively in the outer and the inner membrane. | Tol and Pal are membrane proteins which are located respectively in the outer and the inner membrane. | ||
Tol and Pal proteins interact with each other, forming Tol/Pal complexes. | Tol and Pal proteins interact with each other, forming Tol/Pal complexes. | ||
| - | By acting like press studs binding the inner and outer membranes, thus stabilizing the outer membrane using the peptidoglycan rigidity, the Tol/Pal complexes play a central role to preserve membrane integrity [ | + | By acting like press studs binding the inner and outer membranes, thus stabilizing the outer membrane using the peptidoglycan rigidity, the Tol/Pal complexes play a central role to preserve membrane integrity <sup>[[https://2009.igem.org/Team:Paris/Production_modeling2#References 5]]</sup><sup>[[https://2009.igem.org/Team:Paris/Production_modeling2#References 6]]</sup>. |
[[Image:TolPal Ancored.png|300px|center]] | [[Image:TolPal Ancored.png|300px|center]] | ||
| Line 251: | Line 265: | ||
where x and y are the coordinate of the surface and where the different partial derivatives are depending on the cartesian coordinates of the surface we are evolving on. | where x and y are the coordinate of the surface and where the different partial derivatives are depending on the cartesian coordinates of the surface we are evolving on. | ||
| + | <span/ id="4"> | ||
===Unequal osmotic pressures create small blebbing=== | ===Unequal osmotic pressures create small blebbing=== | ||
| + | |||
| + | |||
Using the equations for membrane deformation, we can compute numerically the shape of the outer membrane at steady state under various assumptions. | Using the equations for membrane deformation, we can compute numerically the shape of the outer membrane at steady state under various assumptions. | ||
| Line 262: | Line 279: | ||
</center> | </center> | ||
| + | <span/ id="5"> | ||
===Tol/Pal accumulates at the basis of nascent blebs=== | ===Tol/Pal accumulates at the basis of nascent blebs=== | ||
| + | |||
The value of the Laplacian operators used to model Tol and Pal diffusions in the membranes (see equations above) depends on the membrane curvature: in non-flat membranes, the "efficiency" of the diffusion depends on the direction. | The value of the Laplacian operators used to model Tol and Pal diffusions in the membranes (see equations above) depends on the membrane curvature: in non-flat membranes, the "efficiency" of the diffusion depends on the direction. | ||
In fact, '''molecules anchored in the outer membrane''', like Pal, '''diffuse less efficiently in regions of negative curvature, and consequently tend to accumulate in these regions'''. | In fact, '''molecules anchored in the outer membrane''', like Pal, '''diffuse less efficiently in regions of negative curvature, and consequently tend to accumulate in these regions'''. | ||
| - | |||
| - | + | Using our computer program, we have been able to experimentally demonstrate this accumulation. | |
| + | The bottom plot presents the accumulation of Pal proteins in the membrane represented on the top plot. | ||
| + | In the bottom plot, the left/right axis represents coordinates along the membrane, whereas the other horizontal axis represent the time evolution of protein concentrations (from time 70 to 100, instead of from 0 to 25 as indicated) | ||
| + | One can show the protein accumulation at coordinates 11 and 43 approximately, corresponding to the regions of negative curvature of the membrane. | ||
| - | + | [[Image:Accumulation2.png|600px|center]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[Image: | + | [[Image:Accumulation3.png|600px|center]] |
| - | |||
| - | + | This result is corroborated by recent experimental findings showing that “negative membrane curvatures [act] as a cue for sub-cellular localization of a bacterial protein” <sup>[[https://2009.igem.org/Team:Paris/Production_modeling2#References 7]]</sup>. | |
| - | |||
| - | |||
| - | ''' | + | Most notably, we can thus assume that Pal molecules accumulate at the basis of blebs. |
| + | This naturally leads to an increase of Tol-Pal complexes formation in these regions. | ||
| + | Lastly, because we additionally assumed that these large complexes diffuse less efficiently than Tol and Pal alone, we finally obtain that '''Tol-Pal complexes accumulate at the basis of the blebs'''. | ||
| + | This is depicted in the following picture. | ||
| - | |||
| - | + | [[Image:Shema_Curvature.png|400px|center]] | |
| - | + | <span/ id="6"> | |
| - | + | ===Rings of accumulated Tol/Pal constricts blebs basis=== | |
| - | |||
| - | + | As explained above, Tol-Pal complexes tend to accumulate at the basis of the blebs, creating a ring of Tol-Pal (if seen from above). | |
| - | + | [[Image:Shema_diffusion.png|400px|center]] | |
| - | |||
| - | + | When Tol-Pal molecules move to the interior of the ring, due to Brownian motion, they narrow the two membranes because they impose to have a constant distance between the two membranes as explained in the picture below: the proteins act like zippers on membranes, as depicted below. This leads to vesicle formation. | |
| - | |||
| + | [[Image:zip.png|400px|center]] | ||
| - | + | <html> | |
| - | + | </div> | |
| - | + | <div id="paris_content_boxtop"> | |
| - | + | </div> | |
| - | + | <div id="paris_content"> | |
| - | + | </html> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ====References==== | ||
| - | + | <ol class="References"> | |
| + | <li> [[Team:Paris/Production_modeling2#1 | ^]]1991 - Lipowsky - The conformation of membranes, ''Nature'', 349(6309):475-481</li> | ||
| + | <li> [[Team:Paris/Production_modeling2#2 | ^]]2008 - Park & Uehara - How bacteria consume their own exoskeletons, ''Microbiol Mol Biol Rev'', 72(2):211-227</li> | ||
| + | <li> [[Team:Paris/Production_modeling2#3 | ^]]1998 - Zhou ''et al'' - On the origin of membrane vesicles in gram-negative bacteria, ''FEMS microbiology letters'', 163(2):223-228 </li> | ||
| + | <li> [[Team:Paris/Production_modeling2#4 | ^]]1987 - Ou-Yang & Helfrich - Instability and deformation of a spherical vesicle by pressure, ''Phys. Rev. Lett.'', 59:2486-2488</li> | ||
| + | <li> [[Team:Paris/Production_modeling2#5 | ^]]2009 - Deatherage ''et al'' - Biogenesis of bacterial membrane vesicles, ''Mol Microbiol'', 72(6):1395-1407 </li> | ||
| + | <li> [[Team:Paris/Production_modeling2#6 | ^]]2005 - Kuehn & Kesty - Bacterial outer membrane vesicles and the host pathogen interaction, ''Genes & Dev'', 19:2645-2655 </li> | ||
| + | <li> [[Team:Paris/Production_modeling2#7 | ^]]2009 - Kumaran & Losick - Negative membrane curvature as a cue for subcellular localization of a bacterial protein. ''PNAS USA'', 106(32):13541-13545 | ||
| + | </li> | ||
| + | </ol> | ||
Latest revision as of 03:55, 22 October 2009
iGEM > Paris > DryLab > Vesicle biophysics Model (vesicle model)
Contents |
DryLab - Vesicles biophysics model
This part aims at investigating an important issue regarding the creation of vesicles:
What is the link between Tol/Pal expression and vesicle formation?
Or, more precisely, can we explain vesicle formation solely by the diffusion of the doubly anchored Tol/Pal complex in the membrane ?
Understanding this connection is instrumental, since our project relies on the hypothesis that an increased rate of vesicle formation can be obtained simply by destabilization of the Tol/Pal complexes.
To answer this question, we developed a biophysical model of the cellular membranes and Tol/Pal complexes, that incorporates outer membrane deformation and Tol/Pal diffusion in membranes by Brownian motion.
Our initial simulation results suggested a three-stage process for vesicle formation.
Thus, in what follows, we decomposed the original main question in three parts:
- Can we explain the formation of small blebs by differences of osmotic pressures between intra- and extra-cellular environments?
- Can we explain the accumulation of Tol/Pal molecules at the basis of nascent blebs?
- Can we explain vesiculation by constriction of blebs basis by accumulated Tol/Pal molecules?
Before answering these questions, we start by describing how we modeled membrane deformation and Tol/Pal diffusion.
The main features of our membrane model is that we take into account the presence of osmotic pressure differences between intra and extra cellular environments and the existence of an outer membrane intrinsic preferred curvature.
The main feature of our Tol/Pal diffusion model is that we take into account that diffusion may happen on non-flat surfaces, and that the Tol/Pal complex is anchored both in the intracellular (Tol) and extracellular (Pal) membranes.
We assumed that the intracellular membrane position is fixed (pushed against the peptidoglycan layer).
Modeling membrane at steady state
The objective of this part is to discuss the existence of an intrinsic curvature of the outer membrane at steady state, and to obtain an estimate of its characteristic parameter.
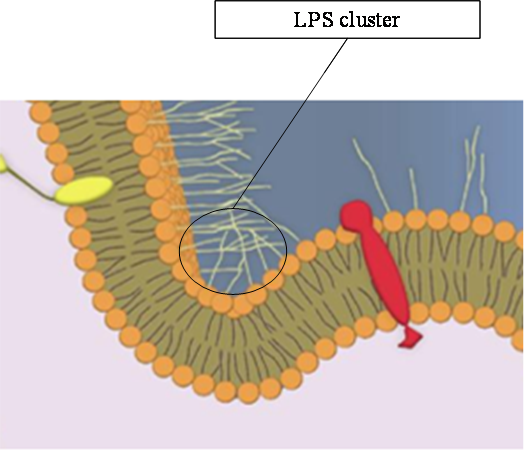
Lipopolysaccharides (LPSs) are important constituents of the E. coli outer membrane.
They possess long polycaccharide chains pointing outwards.
These extracellular sugar extensions create mutual attraction forces that curb the membrane.
This situation is depicted in the figure below.
From a physical point of view, the lipid bilayer behaves like a liquid at 37°c.
To each membrane conformation, one can associate an energy, called the bending energy [1].
This is given by :
where E is the energy of the membrane, Kb and Kg are Bending and Gaussian moduli, Hg is the Gaussian curvature and c0 is a parameter describing the intrinsic curvature of the outer membrane at steady state (ie, minimal energy). The shape of the membrane is described locally by the principal curvature variables c1 and c2 (see [http://en.wikipedia.org/wiki/Principal_curvature here] for details). dS is an infinitesimal surface element.
Under simple assumptions on the shape of E. coli (seen as a cylinder) and assuming that on average the size of the vesicles is the one requiring a minimum of energy, we can using the above relation relate the radius of E. coli r, the mean radius of vesicles r', and the γ0 parameter:
Using r= 300 nm and r’=100 nm (the vesicle size ranges from 25 nm to 175 nm), we estimate the outer membrane intrinsic curvature as
Obtaining an estimation of this parameter is essential to model and simulate the membrane dynamic deformation under osmotic pressure differences as we will see.
Modeling membrane deformation
To obtain a reasonably simple model, we assumed that E. coli cells can be represented as cylinders, as a first approximation. This way, we could develop a 2D model of a transversal section of cells, using cylindrical coordinates.
Because of osmotic pressure differences, we assumed that the intracellular membrane is pushed against the -perfectly circular- peptidoglycan layer. So, only outer membrane deformations have to be modeled.
The difference of osmotic pressure between the intra-cellular environment and the periplasm, and between the periplasm and the extra-cellular environment comes notably from the turnover of peptidoglycan molecules [1]. This hypothesis has been made by Zhou & Doyle [3]
To model membrane deformation, we use the equation proposed by Ou-Yang and Helfrich [4]:
After simplifications, we obtain the following equality.
In this equation, γ is the variable membrane curvature, Δp is the osmotic pression difference, c0 is the membrane intrinsic curvature, and other parameters are as described in Ou-Yang and Helfrich [4].
A formal connection between the membrane curvature γ and the cylindric coordinate variables r and θ can be obtained by the following polar curvature simplification approximation:
where
Lastly, by combining the two equations given above, we obtain the following set of differential equations describing membrane deformations:
Considering that we have low concentrations we can obtain a simpler formula analogue to the perfect gases law:
Finaly we have:
Here we observe that the model is depending of the volume V which will stabilized the equation and totally define all the parameters of the system.
In addition, we decided to model the role of the Tol/Pal system as boundary condition for the system of differential equations : cluster of Tol-Pal are considered as a point with a radius equal to peptidoglycan's one plus the length of the protein. Furthermore, as the surface is closed, we must impose the fact that r(0)=r(2π) to account for closing the vesicle.
Modeling Tol/Pal diffusion
Tol and Pal are membrane proteins which are located respectively in the outer and the inner membrane. Tol and Pal proteins interact with each other, forming Tol/Pal complexes. By acting like press studs binding the inner and outer membranes, thus stabilizing the outer membrane using the peptidoglycan rigidity, the Tol/Pal complexes play a central role to preserve membrane integrity [5][6].
The diffusion of proteins in these lipid bilayers can be modeled by Brownian motion. This diffusion model gives the probability law for the location of Tol and Pal in the membranes.
The following three equations model the Brownian motion of Tol, Pal and Tol/Pal complexes, represented as particles.
In the above equations, CX denotes the concentration of protein X, and DX denotes the constant kinetic coefficient associated to X. The kTolPal and k*TolPal denote the reaction constants for Tol-Pal complexation. In the last equation, Φ is an unknown function representing the Tol-Pal complex motility. However, because the Tol-Pal complex is significantly larger than the other proteins, and is doubly anchored in membranes, we made the assumption that the mobility of Tol-Pal complexes is negligable in comparison to the mobility of isolated Tol and Pal molecules. Stated differently, we set Φ to zero. Lastly, additional constraints on Tol/Pal diffusion have been added to enforce a constant distance between Tol and Pal proteins.
In the above equations, the laplacians capture the non-homogeneous molecular diffusion on non-flat membranes.
Indeed, they link the evolution of protein concentrations with the local shape of the membranes, since we know that on a two dimensional space:
where x and y are the coordinate of the surface and where the different partial derivatives are depending on the cartesian coordinates of the surface we are evolving on.
Unequal osmotic pressures create small blebbing
Using the equations for membrane deformation, we can compute numerically the shape of the outer membrane at steady state under various assumptions. If we assume that Tol/Pal complexes acts like press studs locally imposing a fixed distance between the two membranes, and that their initial distribution in the membrane is not totally homogeneous, then we obtain results of the following type.
- Shape at equilibrium of the outer membrane presenting blebs (red) resulting from osmotic pressure differences and with Tol/Pal complexes locally imposing a fixed distance between the inner (green) and outer membranes.
Tol/Pal accumulates at the basis of nascent blebs
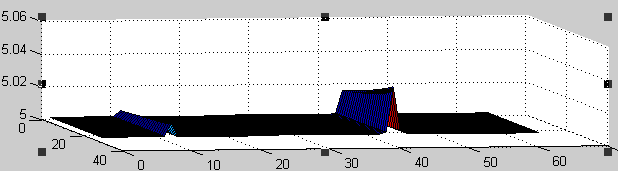
The value of the Laplacian operators used to model Tol and Pal diffusions in the membranes (see equations above) depends on the membrane curvature: in non-flat membranes, the "efficiency" of the diffusion depends on the direction. In fact, molecules anchored in the outer membrane, like Pal, diffuse less efficiently in regions of negative curvature, and consequently tend to accumulate in these regions.
Using our computer program, we have been able to experimentally demonstrate this accumulation. The bottom plot presents the accumulation of Pal proteins in the membrane represented on the top plot. In the bottom plot, the left/right axis represents coordinates along the membrane, whereas the other horizontal axis represent the time evolution of protein concentrations (from time 70 to 100, instead of from 0 to 25 as indicated) One can show the protein accumulation at coordinates 11 and 43 approximately, corresponding to the regions of negative curvature of the membrane.
This result is corroborated by recent experimental findings showing that “negative membrane curvatures [act] as a cue for sub-cellular localization of a bacterial protein” [7].
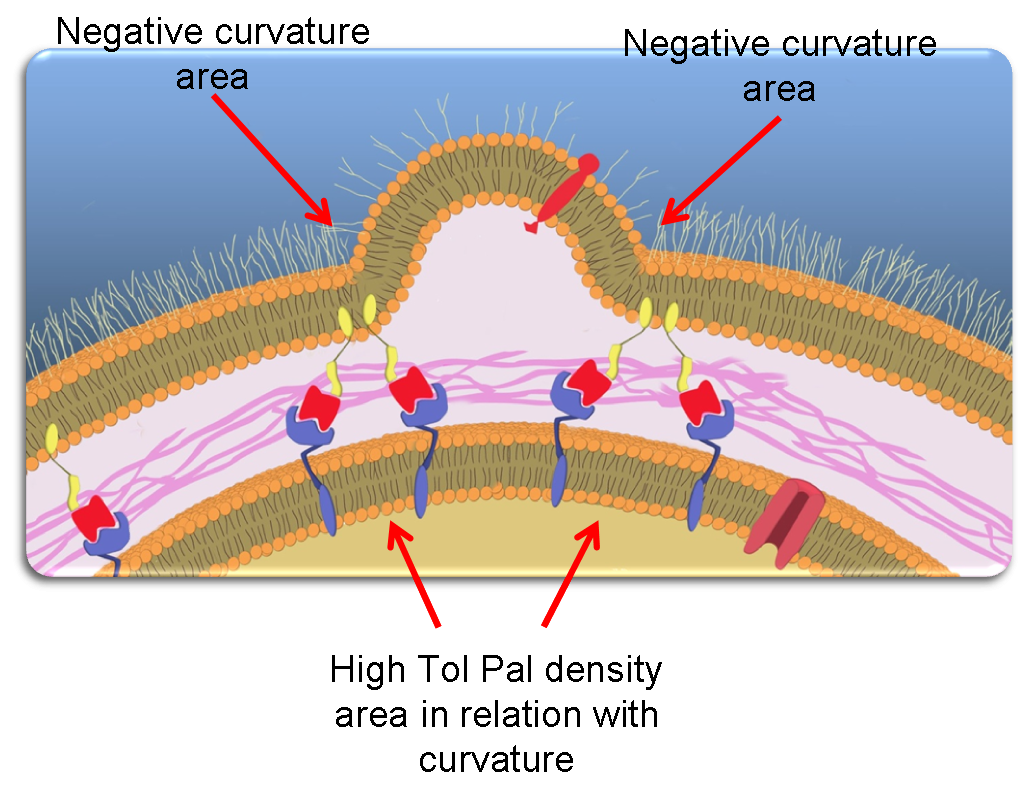
Most notably, we can thus assume that Pal molecules accumulate at the basis of blebs. This naturally leads to an increase of Tol-Pal complexes formation in these regions. Lastly, because we additionally assumed that these large complexes diffuse less efficiently than Tol and Pal alone, we finally obtain that Tol-Pal complexes accumulate at the basis of the blebs. This is depicted in the following picture.
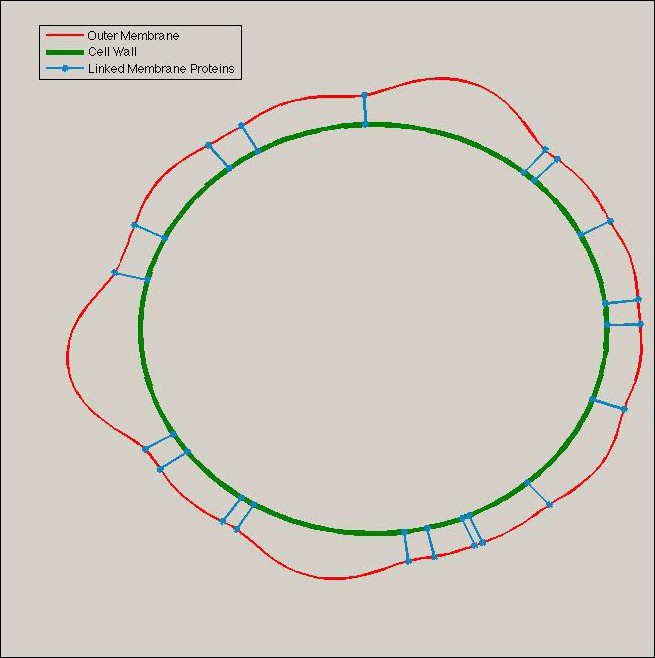
Rings of accumulated Tol/Pal constricts blebs basis
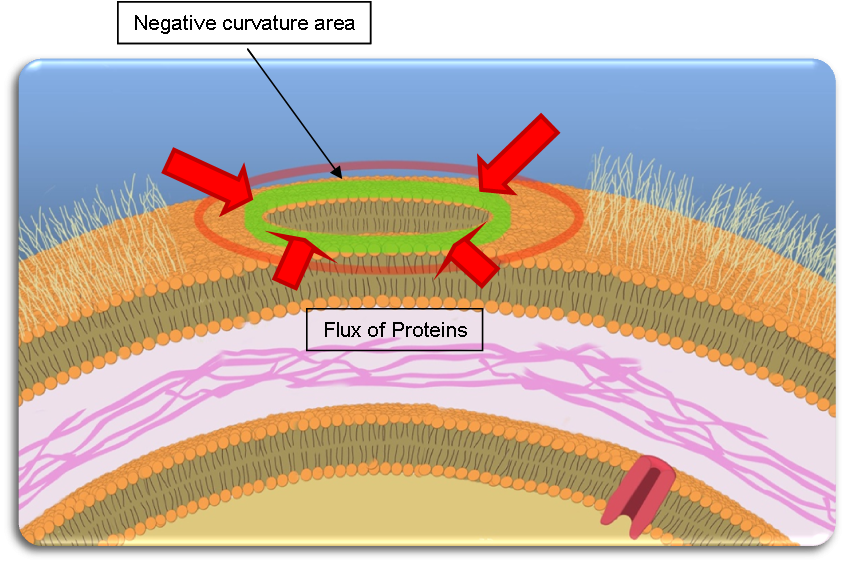
As explained above, Tol-Pal complexes tend to accumulate at the basis of the blebs, creating a ring of Tol-Pal (if seen from above).
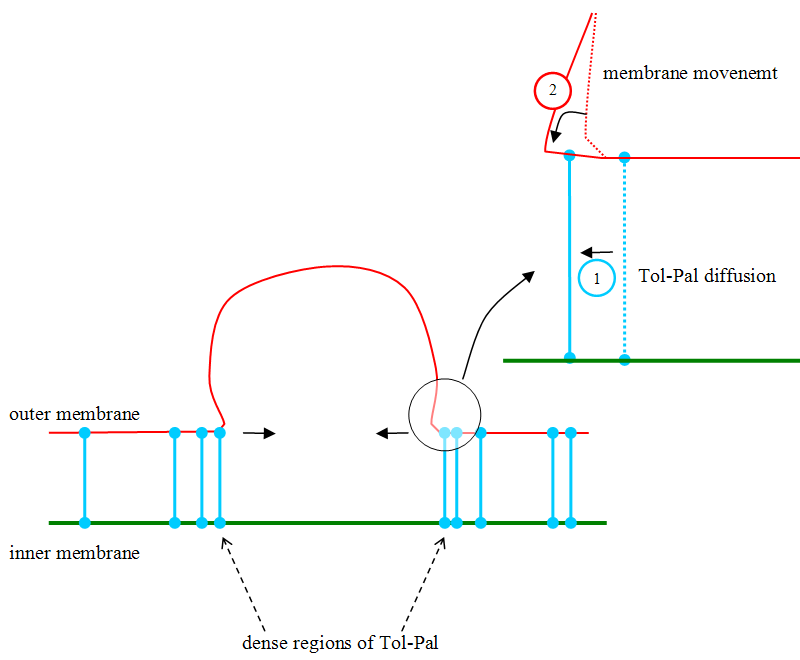
When Tol-Pal molecules move to the interior of the ring, due to Brownian motion, they narrow the two membranes because they impose to have a constant distance between the two membranes as explained in the picture below: the proteins act like zippers on membranes, as depicted below. This leads to vesicle formation.
References
- ^1991 - Lipowsky - The conformation of membranes, Nature, 349(6309):475-481
- ^2008 - Park & Uehara - How bacteria consume their own exoskeletons, Microbiol Mol Biol Rev, 72(2):211-227
- ^1998 - Zhou et al - On the origin of membrane vesicles in gram-negative bacteria, FEMS microbiology letters, 163(2):223-228
- ^1987 - Ou-Yang & Helfrich - Instability and deformation of a spherical vesicle by pressure, Phys. Rev. Lett., 59:2486-2488
- ^2009 - Deatherage et al - Biogenesis of bacterial membrane vesicles, Mol Microbiol, 72(6):1395-1407
- ^2005 - Kuehn & Kesty - Bacterial outer membrane vesicles and the host pathogen interaction, Genes & Dev, 19:2645-2655
- ^2009 - Kumaran & Losick - Negative membrane curvature as a cue for subcellular localization of a bacterial protein. PNAS USA, 106(32):13541-13545
 "
"