Team:Heidelberg/Project Highlights
From 2009.igem.org
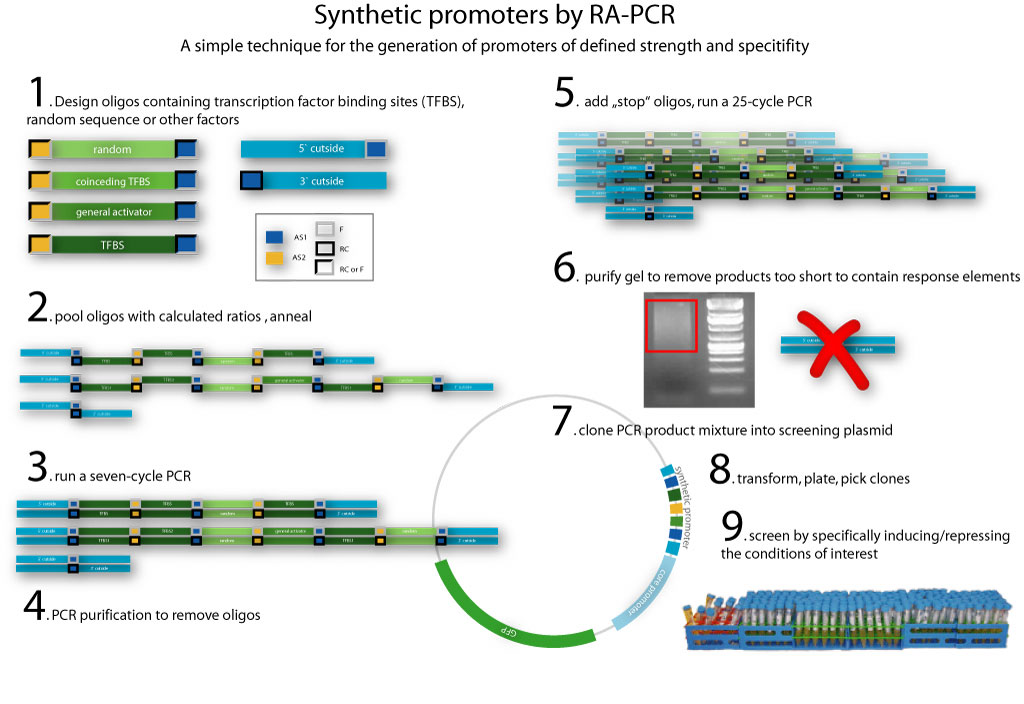
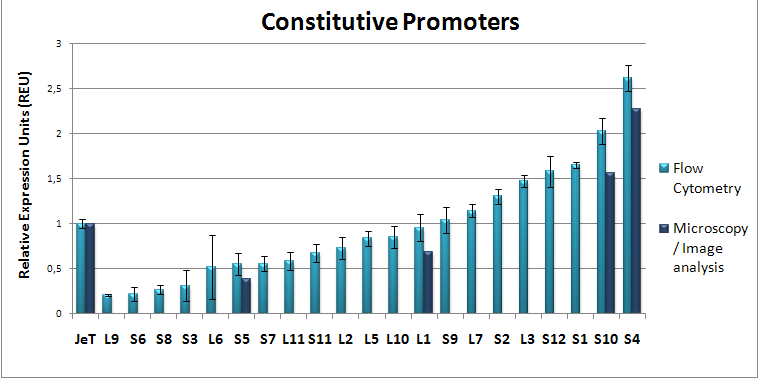
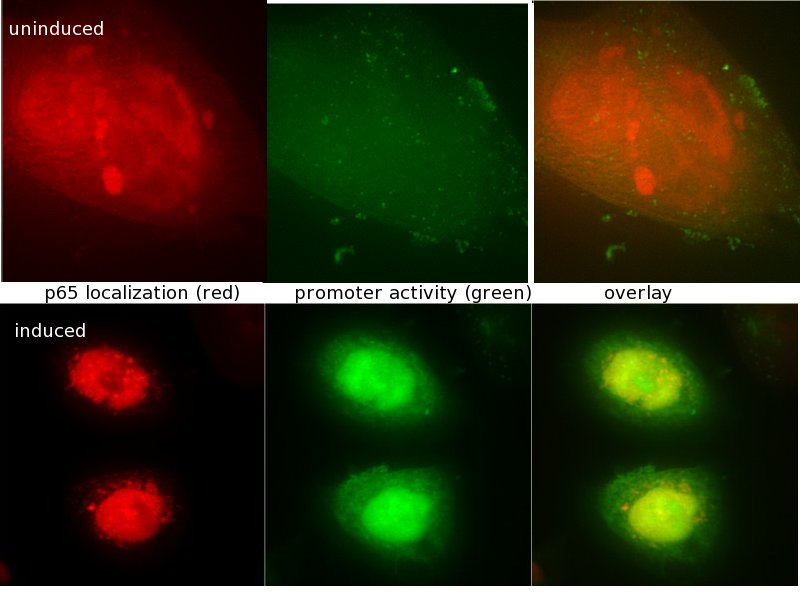
Project HighlightsWe are able to predict functional mammalian promoter sequencesWe created HEARTBEAT, a model portfolio based upon genome-wide bioinformatic analysis and fuzzy logic. We used these tools to predict promoter sequences. Then, we synthesized such sequences. We found that promoters most closely matching the model predictions work, whereas others don't. Read more about these results on the "HEARTBEAT database" page. We also present a GUI which enables you to design the promoter of your needs.  HEARTBEAT describes the spatial distribution of Transcription Factor Binding Sites along a mammalian promoter. The diagram on the top shows this distribution for SREBP. HEARTBEAT also describes Transcription Factors co-occurring with a given TF, which, for SREBP, is Sp1. We placed SREBP and Sp1 Binding sites according to the predicted distribution and found only promoters which resemble the distribution closest to be induced by SREBP (see small diagram). Other promoter sequences are not functional We have created a functional biochemical synthesis method for the generation of promoters librariesWe developed RA-PCR, a biochemical method for the generation of randomized promoter libraries. We created a library of constitutive promoters, a NF-κB responsive promoter which underwent extensive characterization and some other regulated promoters. We have developed novel standards for measurement of promoters in mammalian cellsApplying both qRT-PCR and flow cytometry, we developed two new relative units in analogy to bacterial RPU for use in mammalians: Relative Expression Units and Relativeb Mammalian Promoter Units. We apply these units to the characterization of our promoters, as well as an existing [http://partsregistry.org/Part:BBa_I712004 promoter from the registry].
4 RFCs, well characterized partsWe stick with the standards required by synthetic biology and submit all the methods and units we developed as RFCs (Request for Comments):
|
 "
"