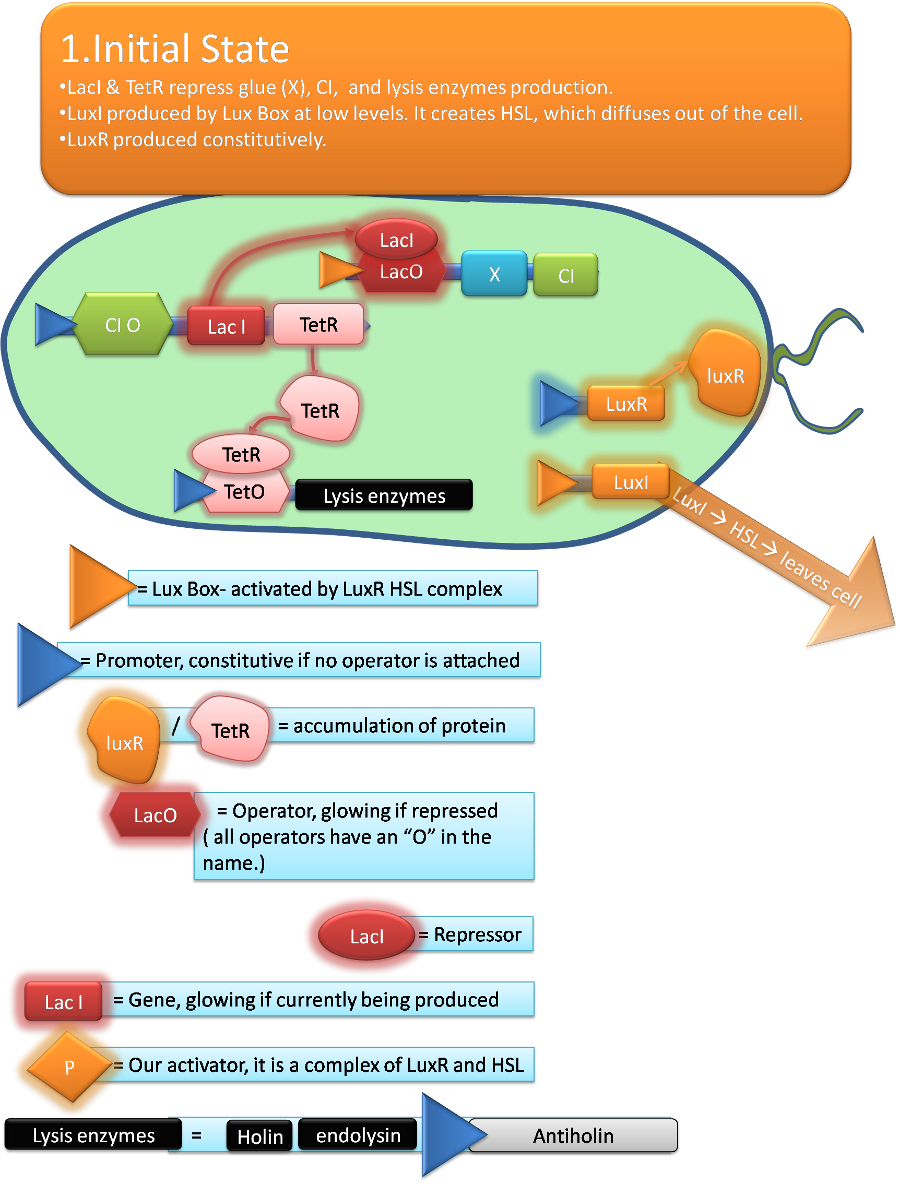
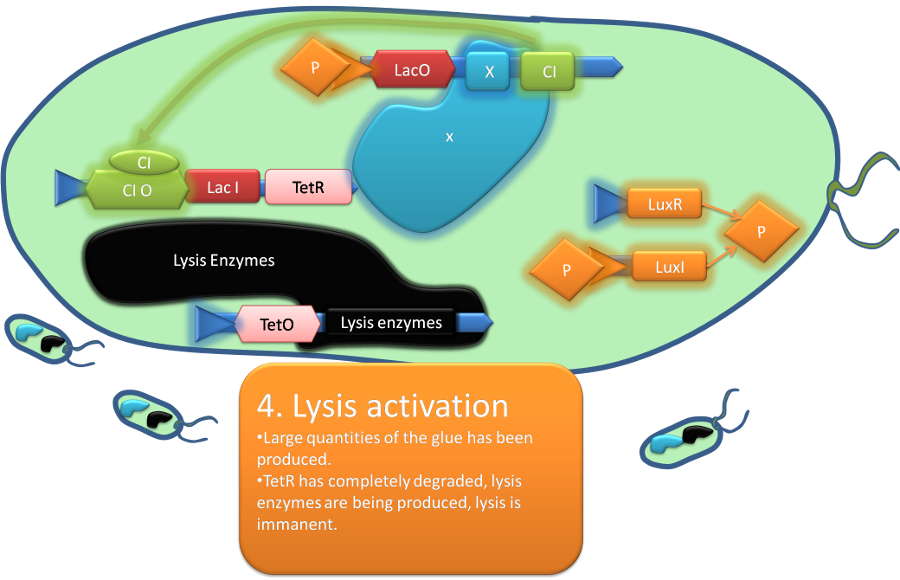
Team:Aberdeen Scotland/Project
From 2009.igem.org
University of Aberdeen - Pico Plumber
Contents |
Pico Plumber: A Project Overview
Introduction
Every day millions of litres of water are lost from leaky pipes all throughout Britain[1]. The London Assembly alone reported a loss of nearly a billion litres of water for the region in 2003/2004[2,3]. Similar problems are experienced by the Oil and Sewage industries where leaky pipes not only translate into a loss of revenue, but also put strain on the environment.
With this in mind we decided to explore ways in which synthetic biology can be used to address this and comparable problems. We concluded that the most useful solution would be one that can detect microscopic leaks by itself and seal them.
In order to achieve this we identified a number of characteristics we wanted our Bacteria to display. Bacteria have first to detect the breach and swim towards it, so that they can repair it. In order to make the bacteria move towards the leaking site, we release aspartate from the leak, so that the bacteria are attracted and swim to it by means of chemotaxis.
The bacteria have to produce the glue only when close to the breach; otherwise, they could stick to each other on their way to the leaking site, and the breach would not be repaired. Therefore, they have to sense the moment when thy have reached the leaking site. To do this, we make use of quorum sensing, a mechanism present in some microorganisms by which a bacterium can detect the presence of other bacteria in the neighborhood. Since all bacteria will swim towards the leaking site, the density of bacteria will increase substantially close to the leak. Hence, by the quorum sensing signal, bacteria "know" that they have reached the leaking site, and therefore, they can start producing the glue.
In order to release the glue, so that the hole in the pipe can be repaired, we make the bacteria lyse after a certain time interval, during which they have produced a sufficient amount of the glue proteins. Since developing a fully functional version of this that can be used in industry would be a task far beyond the scope of an undergraduate summer project, our aim was to develop a proof of concept for the various elements of the system, using a combination of biological and computational approaches, under favorable conditions and upon success to develop them further.
Project and Wiki overview
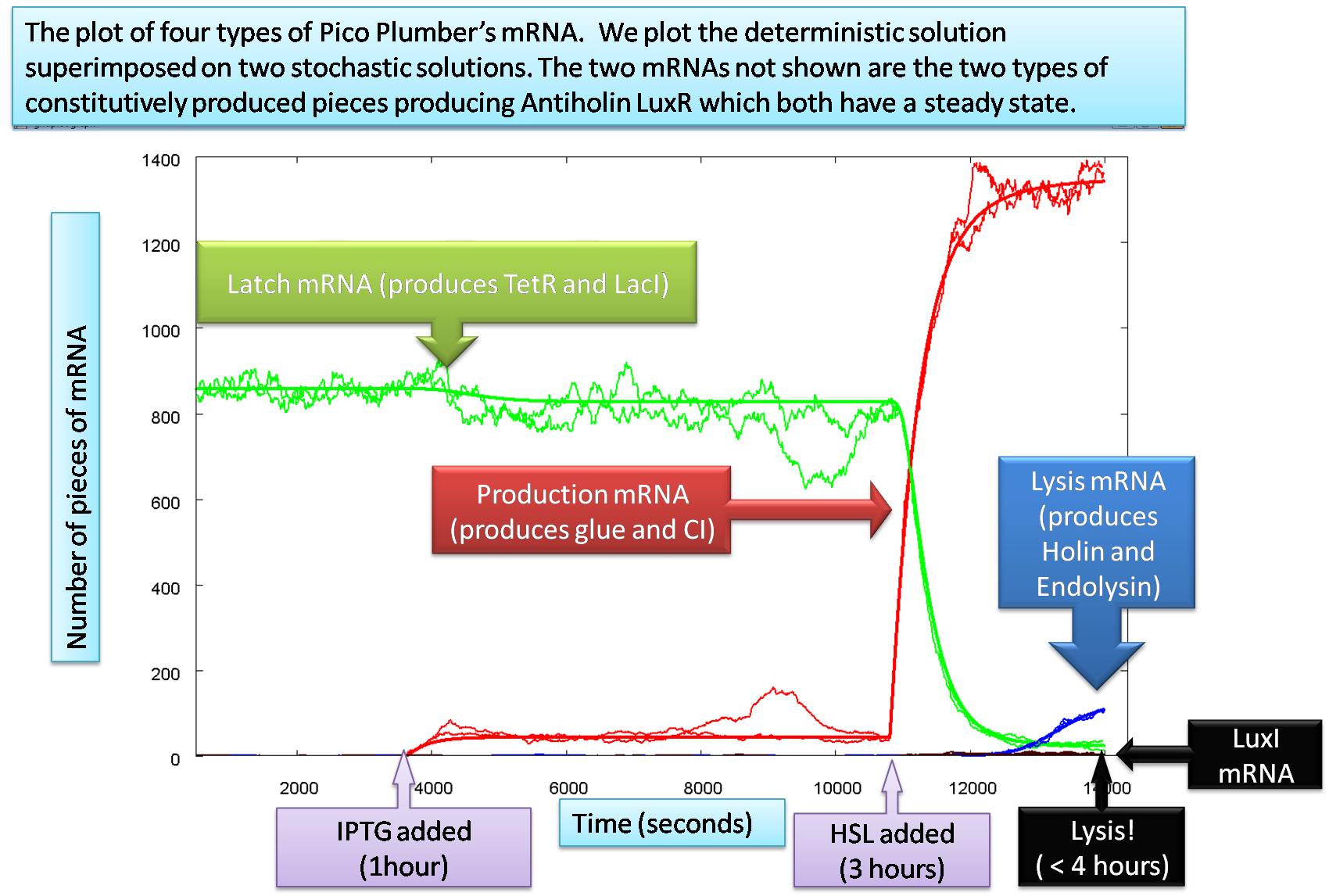
Throughout the summer, we have created models guiding the biology. We simulated the numbers of each of our 6 individual mRNAs, 10 proteins and complexes at every step of 6 hour simulation time with the help of ordinary differential equations (ODEs) both deterministically and stochastically, an example graph is shown here:
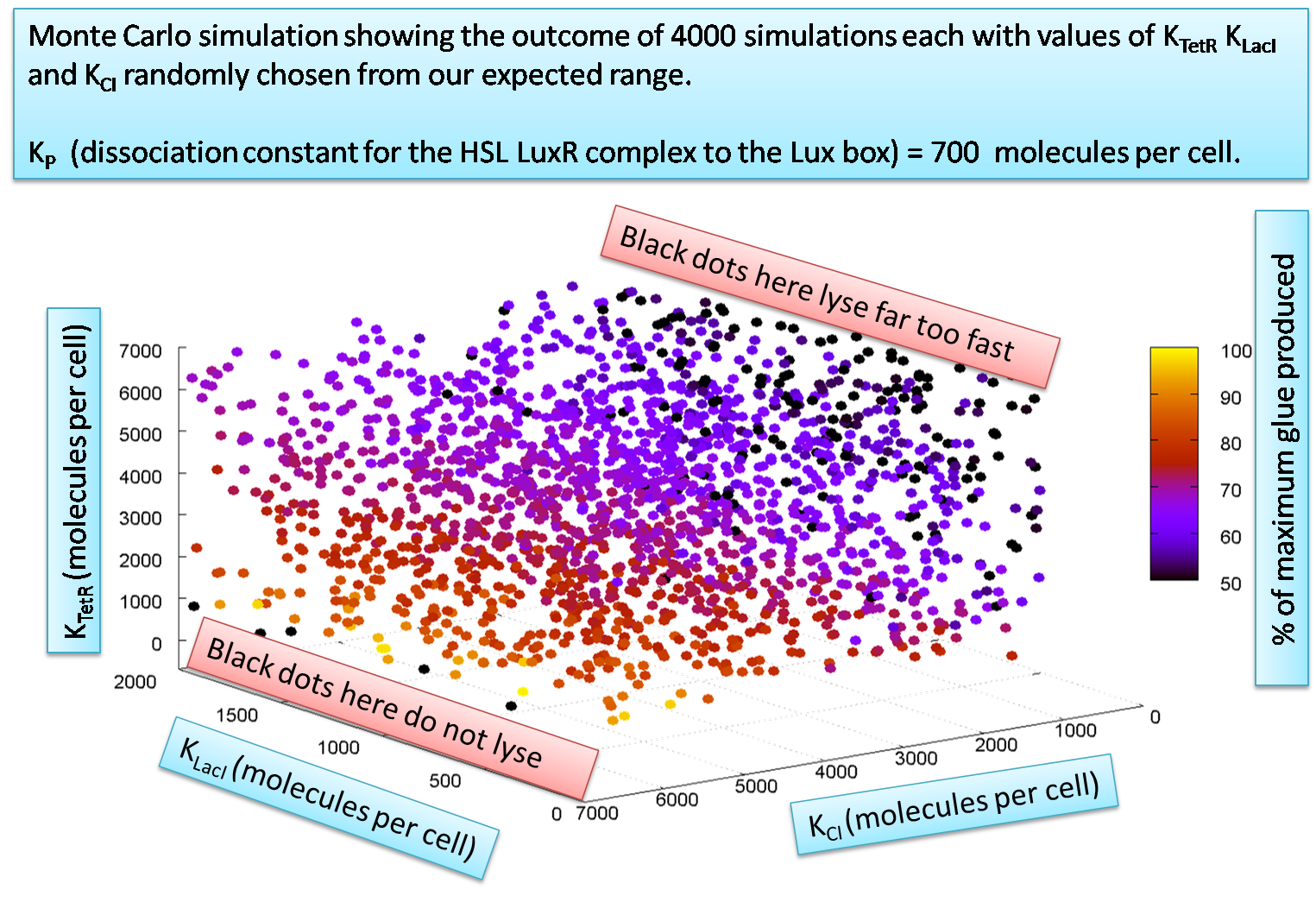
The equations contain approximately 30 parameters which the system is sensitive to. One type of parameter, that the system is particularly dependant apon, are the dissociation constants used in the Hill input functions relevant for the ODEs. Here, we show how we test out sensitivities to 2 different dissociation constants simultaneously
We explore 3 parameters simultaneously using Monte Carlo simulations as in the following graph:
More information on this process can be found in system investigation.
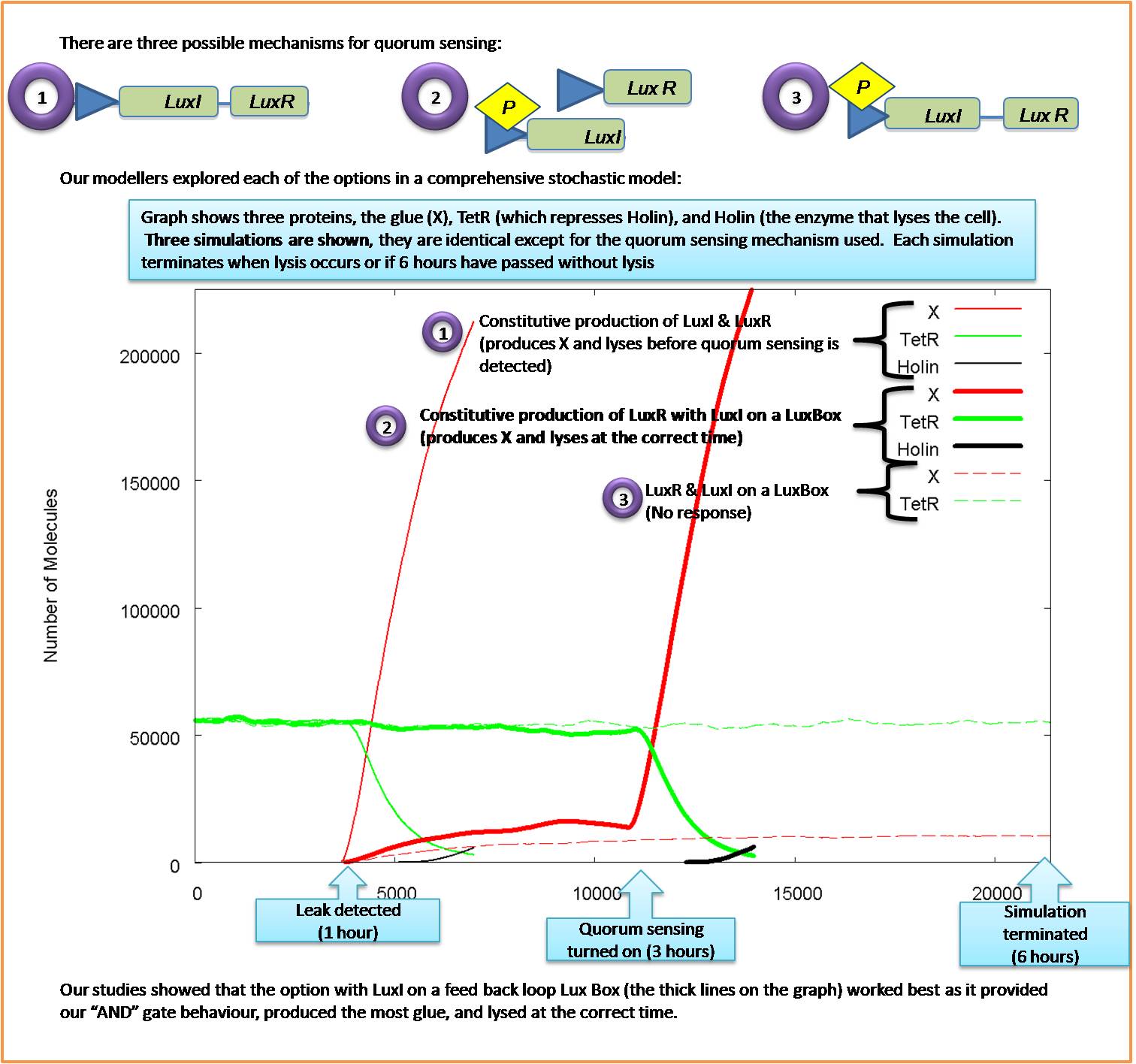
We looked at three possible options for our quorum sensing input and decided which one fullfilled our requirements
We also simulate chemotaxis in E. Coli using MATLAB:
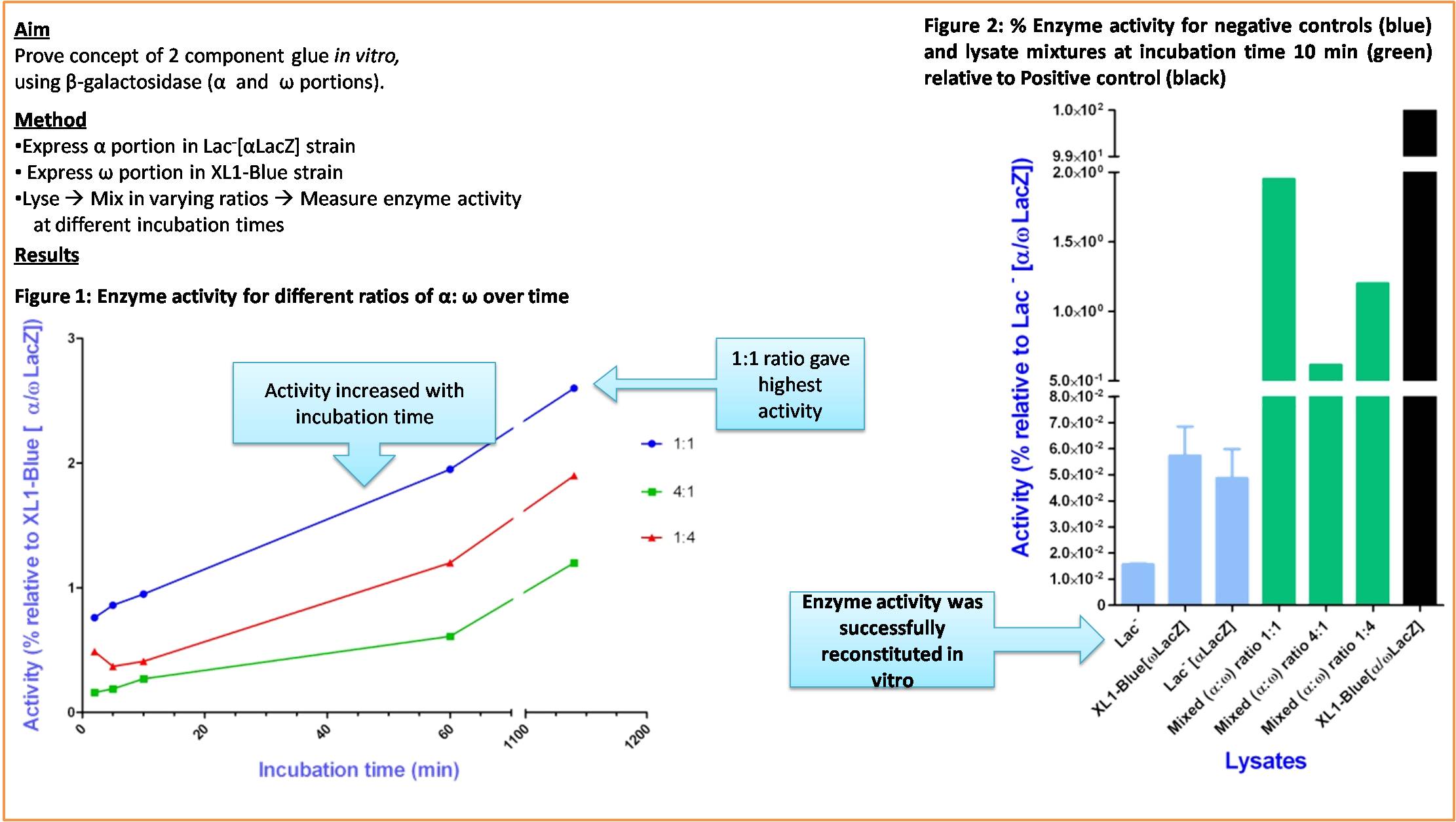
We proved the concept of a two part glue recombining after lysis and becoming active
And finally, here are the 6 biobricks which we created
Scientific summary
Synthetic biology approaches are being used in a novel iGEM project that will engineer E. coli to detect and repair leaks and corrosion in pipes. The bacterium will respond by migrating to the site of the leak and plugging the breach using a protein-based glue. This solution could be used to seal leaks in pipes in inaccessible places, for example within a nuclear power station cooling system, or on a space station. The novelty of the biological engineering lies in the implementation of a two-component glue to seal the pipe, analogous to an epoxy resin that is only activated when two components, an adhesive and hardener, are mixed. In this project E. coli is engineered to synthesise the glue components (tropoelastin and lysyl oxidase) in different cellular compartments, which are then mixed following autolysis of the bacterium. The relative timings of the elastin glue synthesis and autolysis steps are carefully controlled, thus ensuring regulated lysis, but only after tropoeleastin/lysyl oxidase synthesis.
To achieve these aims, E. coli will be engineered to respond to an inducer molecule (IPTG) released from the site of a pipe breach. In this project, our model pipes will have a surface coating of the inducer and the attractant aspartate, which are only released into the pipe in the case of a breach in the wall. Once the inducer IPTG is sensed by the bacterium, lacI repression will be relieved, inducing expression of tropoelastin (adhesive) within the E. coli, and the enzyme lysyl oxidase (the adhesive hardener) on the cell surface. This will be achieved by expressing lysyl oxidase as a translational fusion with the outer membrane protein OmpX. Motile E. coli will be used to allow chemotactic migration of the tropoelastin-expressing E. coli to the site of the pipe breach.
The expression of tropoelastin within the cytoplasm, and lysyl oxidase on the cell surface, will maintain the glue components in separate compartments. Their mixing at the site of pipe breach will be achieved by regulated autolysis, triggered by the lytic peptide T4 holin, initiating a cross-linking reaction and the formation of elastin. However, holin expression will be delayed relative to that of tropoelastin and lysyl oxidase, to allow the manufacture of glue components before cell lysis and glue mixing is initiated. This temporal delay will be achieved by using IPTG to induce a molecular inverter element that switches off a repressor of holin production. Slow decay in the levels of this repressor will trigger eventual holin synthesis thereby allowing cell lysis and tropoelastin/lysyl oxidase mixing.
This project is designed in a modular way; individual components will be mathematically modelled and their design improved, before being built and tested. The separate testing of these modules will ensure that the project will not be compromised should any one module perform unpredictably. At the same time, the dependence of the project on the carefully staged expression of different gene modules will ensure a challenging research project for our theoreticians and biologists alike, who will work together to improve and implement the design of a novel two-component microbial glue for use in self-healing pipes.
Conclusions
Our project began by modeling three different designs and finding the optimum one. Our modelling then extended to cover the three main topics which were the internal system, the quorum sensing system and the chemotactic system. Our investigations led to the following successes:
- Guiding the biology in choosing the best design for implementing the pico plumber.
- Effectively modelled the behavior of mRNA transcription and protein translation, using both deterministic and stochastic methods.
- Analysis of all of the important parameters in the deterministic and stochastic systems which let us investigate the robustness of our system and maximise it.
- Created a probabilistic chemotaxis model, which reproduced the behaviour of E.coli cells as accurately as possible. The model took into account both movement when no chemotactic gradient was sensed by the e.coli and when they had sensed a chemotactic gradient and were moving up it.
- From our models, we concluded that the original design would not work, since it would turn on it's own quorum sensing. We then came up with two alternative designs for the quorum sensing module. We then tested them and found one which produced the required response and was far more robust to parameter changes than the original design.
- Combined the data from the amended stochastic model with the chemotaxis model to create a combined population model to investigate the complete system behaviour.
- We proposed that a parameter database be created, so that modeling done by future iGEM teams could be done faster, more easily and more accurately by having access to a central source of biological parameters.
The theoretical modelling guided the design of the Pico Plumber and during the summer significant progress was made in the construction of all of the key modules. A number of these modules were tested as was the concept of creating a two component glue. The biobricks we created can be found HERE and the full list of assembled pieces can be found in WET LAB.
The main achievements of the WetLab Effort are as follows:
- We constructed and submitted 6 new BioBricks to registry. These are (BBa_K182001, BBa_K182005, BBa_K182100, BBa_K182101, BBa_K18102, BBa_K182200.)
- We constructed and tested the BioBrick BBa_K182005 (integral part of the LacI latch) and demonstrated In vivo expression of the repressor molecule as well as lifting of repression.
- We constructed and tested BioBrick BBa_K182101 - pLux-Lac Hybrid with GFP Reporter and Double Terminator, a promoter regulatable by quorum sensing and lacI. We demonstrated the part has functional promoter activity, which is regulatable by IPTG.
- We successfully demonstrated the in vitro restoration of the activity of Enzymes (using Beta-Galactosidase) after cell lyses which is an integral part of our System in regards to creating the Adhesive.
- We submitted a large experience section for all the biobricks we used. Most notably BBa_J37032. In total we reviewed 21 BioBrick’s.
 Back to Home Back to Home
|
Continue to Parameter Database Proposal 
|
References
[1] http://thescotsman.scotsman.com/uk/Massive-scale-of-UK-water.5514624.jp
[2] http://news.bbc.co.uk/1/hi/england/london/4330721.stm
[3] http://www.thameswater.co.uk/cps/rde/xchg/corp/hs.xsl/6869.htm
 "
"