Team:BCCS-Bristol/Project
From 2009.igem.org
| (30 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:BCCS-Bristol/Header}} | {{:Team:BCCS-Bristol/Header}} | ||
| - | == | + | <html> |
| + | <center> | ||
| + | <img style="width:750px" src="https://static.igem.org/mediawiki/2009/thumb/e/ed/BCCS_igem_banner.png/800px-BCCS_igem_banner.png" > | ||
| + | </center> | ||
| + | </html> | ||
| - | |||
| - | + | {| class="panel" align = "center" width = "60%" | |
| + | |- | ||
| + | |width="25%" padding="3px"| <html><center><a href="https://2009.igem.org/Team:BCCS-Bristol/Project/Wet_lab"><img src="https://static.igem.org/mediawiki/2009/b/b6/BCCS_Wetlabl_button.jpg"></a></center></html> | ||
| + | |width="25%" padding="3px"| <html><center><a href="https://2009.igem.org/Team:BCCS-Bristol/Project/Modelling"><img src="https://static.igem.org/mediawiki/2009/4/48/BCCS_Modelling_button.jpg"></a></center></html> | ||
| + | |- | ||
| + | |width="50%"|<center>[[Team:BCCS-Bristol/Project/Wet_lab|Wet Lab]]</center> | ||
| + | |width="50%"|<center>[[Team:BCCS-Bristol/Project/Modelling|Modelling]]</center> | ||
| + | |- | ||
| + | |Click above to read more about our wet-lab work. | ||
| + | |Click above to read more about our team's modelling approach and the simulations we performed to assess the effectiveness of OMV based communication. | ||
| + | |- | ||
| + | |} | ||
| - | |||
| - | |||
| - | == | + | == Project description == |
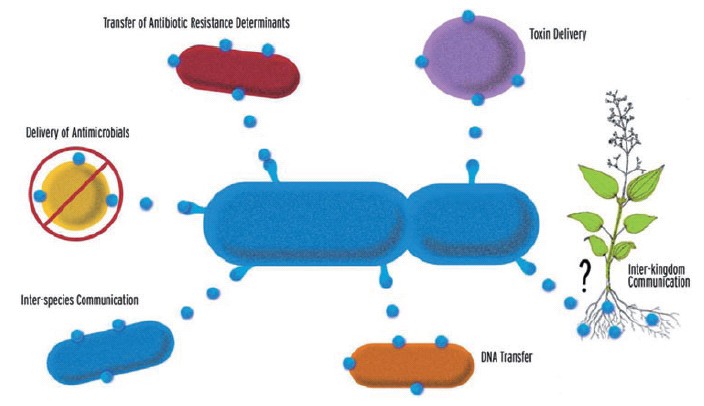
| - | + | [[Image:BCCS-Bristol_vesicles.jpg|thumb|The known and hypothesized roles of Gram-negative OMVs [4]]] | |
| - | + | Our aim was to construct a system for directed delivery of proteins into cells by outer membrane vesicle (OMV) protein secretion. Gram-negative bacteria naturally produce outer member vesicles (OMVs): spherical, bilayered proteolipids from 20-200nm in diameter. OMVs can carry outer membrane, periplasmic and cytoplasmic proteins, DNA, RNA and other factors associated with virulence. They have been implicated in the delivery of toxins to host cells, in the transfer of proteins and genetic materials between bacterial cells and in cell-to-cell signalling [2]. Synthetic biology offers the potential to engineer these mechanisms for human benefit, for example, the injection of toxic cocktails of proteins into cancerous cells or the replacement of proteins missing as a result of genetic defects [3]. These systems may be further enhanced by localised delivery of OMVs, for example, via the control of magnetotactic bacteria using external magnetic fields [5]. | |
| - | + | We designed [https://2009.igem.org/Team:BCCS-Bristol/Project/Wet_lab/BioBricks Biobricks] with the potential to allow the secretion of any protein in OMVs via fusion with novel, non-toxic partners known to be enhanced in OMVs, like FhuA and OsmE proteins. We hope that the Biobricks will become widely used as a simple and effective means of protein secretion, whilst also conveying the unique engineering advantages of vesicular encapsulation. In order to allow easy protein fusions we designed and created a new protein fusion assembly standard, [http://hdl.handle.net/1721.1/49505 RFC44], that provides the basis of a solution to a biobrick-friendly protein fusion mechanism that supports the current favorite assembly standard 10 (RFC10) and hence allows utilization of most of the current biobricks database. | |
| + | |||
| + | |||
| + | The modelling team continued work on [https://2009.igem.org/Team:BCCS-Bristol/BSim BSim], the agent-based modelling framework started by last year's Bristol team. A slew of new features were added and the package was made much more extensible to the point that we believe BSim has a realistic chance of becoming the tool of choice for simulation of interacting bacterial populations. BSim was used to study simultaneous delivery of proteins into cells via packaging in OMVs and several other scenarios unrelated to vesicles, demonstrating its flexibility. | ||
| + | |||
| + | |||
| + | |||
| + | == References == | ||
| + | |||
| + | [1] https://2008.igem.org/Team:BCCS-Bristol/Modeling, true 30th July 2009 | ||
| + | |||
| + | [2] Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli, [http://dx.doi.org/doi:10.1002/pmic.200700196 doi:10.1002/pmic.200700196] | ||
| + | |||
| + | [3] Engineered Bacterial Outer Membrane Vesicles with Enhanced Functionality, [http://dx.doi.org/doi:10.1016/j.jmb.2008.03.076 doi:10.1016/j.jmb.2008.03.076] | ||
| + | |||
| + | [4] Special delivery: vesicle trafficking in prokaryotes, [http://dx.doi.org/doi:10.1111/j.1365-2958.2006.05272.x doi:10.1111/j.1365-2958.2006.05272.x] | ||
| + | |||
| + | [5] Controlled manipulation and actuation of micro-objects with magnetotactic bacteria, [http://dx.doi.org/doi:10.1063/1.2402221 doi:10.1063/1.2402221] | ||
Latest revision as of 03:54, 22 October 2009
iGEM 2009

 |  |
| Click above to read more about our wet-lab work. | Click above to read more about our team's modelling approach and the simulations we performed to assess the effectiveness of OMV based communication. |
Project description
Our aim was to construct a system for directed delivery of proteins into cells by outer membrane vesicle (OMV) protein secretion. Gram-negative bacteria naturally produce outer member vesicles (OMVs): spherical, bilayered proteolipids from 20-200nm in diameter. OMVs can carry outer membrane, periplasmic and cytoplasmic proteins, DNA, RNA and other factors associated with virulence. They have been implicated in the delivery of toxins to host cells, in the transfer of proteins and genetic materials between bacterial cells and in cell-to-cell signalling [2]. Synthetic biology offers the potential to engineer these mechanisms for human benefit, for example, the injection of toxic cocktails of proteins into cancerous cells or the replacement of proteins missing as a result of genetic defects [3]. These systems may be further enhanced by localised delivery of OMVs, for example, via the control of magnetotactic bacteria using external magnetic fields [5].
We designed Biobricks with the potential to allow the secretion of any protein in OMVs via fusion with novel, non-toxic partners known to be enhanced in OMVs, like FhuA and OsmE proteins. We hope that the Biobricks will become widely used as a simple and effective means of protein secretion, whilst also conveying the unique engineering advantages of vesicular encapsulation. In order to allow easy protein fusions we designed and created a new protein fusion assembly standard, [http://hdl.handle.net/1721.1/49505 RFC44], that provides the basis of a solution to a biobrick-friendly protein fusion mechanism that supports the current favorite assembly standard 10 (RFC10) and hence allows utilization of most of the current biobricks database.
The modelling team continued work on BSim, the agent-based modelling framework started by last year's Bristol team. A slew of new features were added and the package was made much more extensible to the point that we believe BSim has a realistic chance of becoming the tool of choice for simulation of interacting bacterial populations. BSim was used to study simultaneous delivery of proteins into cells via packaging in OMVs and several other scenarios unrelated to vesicles, demonstrating its flexibility.
References
[1] https://2008.igem.org/Team:BCCS-Bristol/Modeling, true 30th July 2009
[2] Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli, [http://dx.doi.org/doi:10.1002/pmic.200700196 doi:10.1002/pmic.200700196]
[3] Engineered Bacterial Outer Membrane Vesicles with Enhanced Functionality, [http://dx.doi.org/doi:10.1016/j.jmb.2008.03.076 doi:10.1016/j.jmb.2008.03.076]
[4] Special delivery: vesicle trafficking in prokaryotes, [http://dx.doi.org/doi:10.1111/j.1365-2958.2006.05272.x doi:10.1111/j.1365-2958.2006.05272.x]
[5] Controlled manipulation and actuation of micro-objects with magnetotactic bacteria, [http://dx.doi.org/doi:10.1063/1.2402221 doi:10.1063/1.2402221]
 "
"