Team:Cambridge/Project/Melanin
From 2009.igem.org
Categories :
Project :
-
Overview
Sensitivity Tuner
--- Characterisation
--- Modelling
Colour Generators
--- Carotenoids (Orange/Red)
--- Melanin (Brown)
--- Violacein (Purple/Green)
The Future
Safety
Notebook :
Team Logistics :
Melanin Pigment
Background
Melanin Production
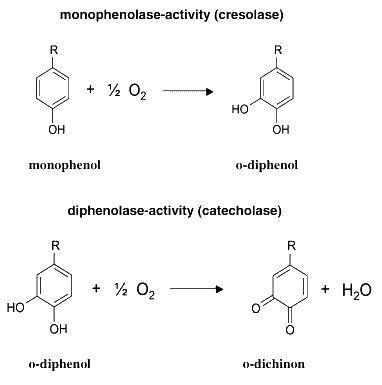
The MelA gene codes for a tyrosinase. Tyrosinases catalyze two reactions, as described in the figure below. Melanin is a macromolecular compound produced by the polymerization of the quinone product of the second reaction, and has a characteristic brown colour.
From H. Claus and H. Decker, Bacterial tyrosinases, Syst. Appl. Microbiol. 29 (2006), pp. 3–14. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B7GVX-4H21K91-1&_user=1495569&_coverDate=01%2F24%2F2006&_fmt=full&_orig=search&_cdi=20442&view=c&_acct=C000053194&_version=1&_urlVersion=0&_userid=1495569&md5=de451c5e1d1d18ad12b7e39b10b80408&ref=full]
MelA
Our MelA gene is from Rhizobium etli. Further, it is a mutant; it has a C to T substitution at the 1,000th nucleotide, which creates a Proline to Serine mutation that reduces the amount of time before melanin production is visible. (Santos, C. N., and G. Stephanopoulos. 2008. Melanin-based high-throughput screen for L-tyrosine production in Escherichia coli. Appl. Environ. Microbiol. 74:1190-1197 [http://aem.asm.org/cgi/reprint/74/4/1190]) The plasmid was provided by Christine Sanntos from the lab of G. Stephanopoulos to Duncan Rowe under the materials transfers agreement.
Action plan of our team
Our action plan is as follows:
- 1. Test for melanin production
- 2. Isolate MelA gene in biobrick form
- 3. Integrate Mel biobrick into system (e.g amplification of logic gate system)
Design
Constructing the BioBrick
Biobrick
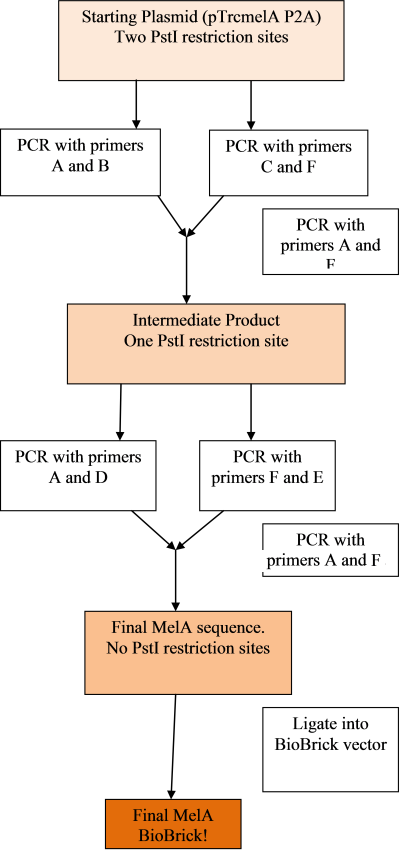
Our aim is to make the MelA gene into a biobrick as follows:
In order to do so, we had to remove forbidden restriction sites within the gene using primers and the in-fusion PCR technique. Primers were ordered as shown by the image below, to add the prefix and suffix and change the PstI restriction site from CTGCAG to CTGCAA:
The PCR's will be done in the following steps, in order to remove both sites and prepare the gene as a biobrick:
Characterisation
Successful Pigment Production
In order to produce pigment, bacteria transformed with pTRCmelA must be plated on media supplemented with copper and tyrosine. Supplementation with copper is necessary as copper is a cofactor for the tyrosinase, and tyrosine is a precursor to melanin biosynthesis. (N. Cabrera-Valladares, A. Martínez, S. Piñero, V.H. Lagunas-Muñoz, R. Tinoco and R. de Anda et al., Expression of the melA gene from Rhizobium etli CFN42 in Escherichia coli and characterization of the encoded tyrosinase, Enzyme Microb Technol 38 (6) (2006), pp. 772–779 [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TG1-4H21NC4-4&_user=1495569&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_searchStrId=972838039&_rerunOrigin=scholar.google&_acct=C000053194&_version=1&_urlVersion=0&_userid=1495569&md5=3a4e939d82057cc796749e16cab13a3b])
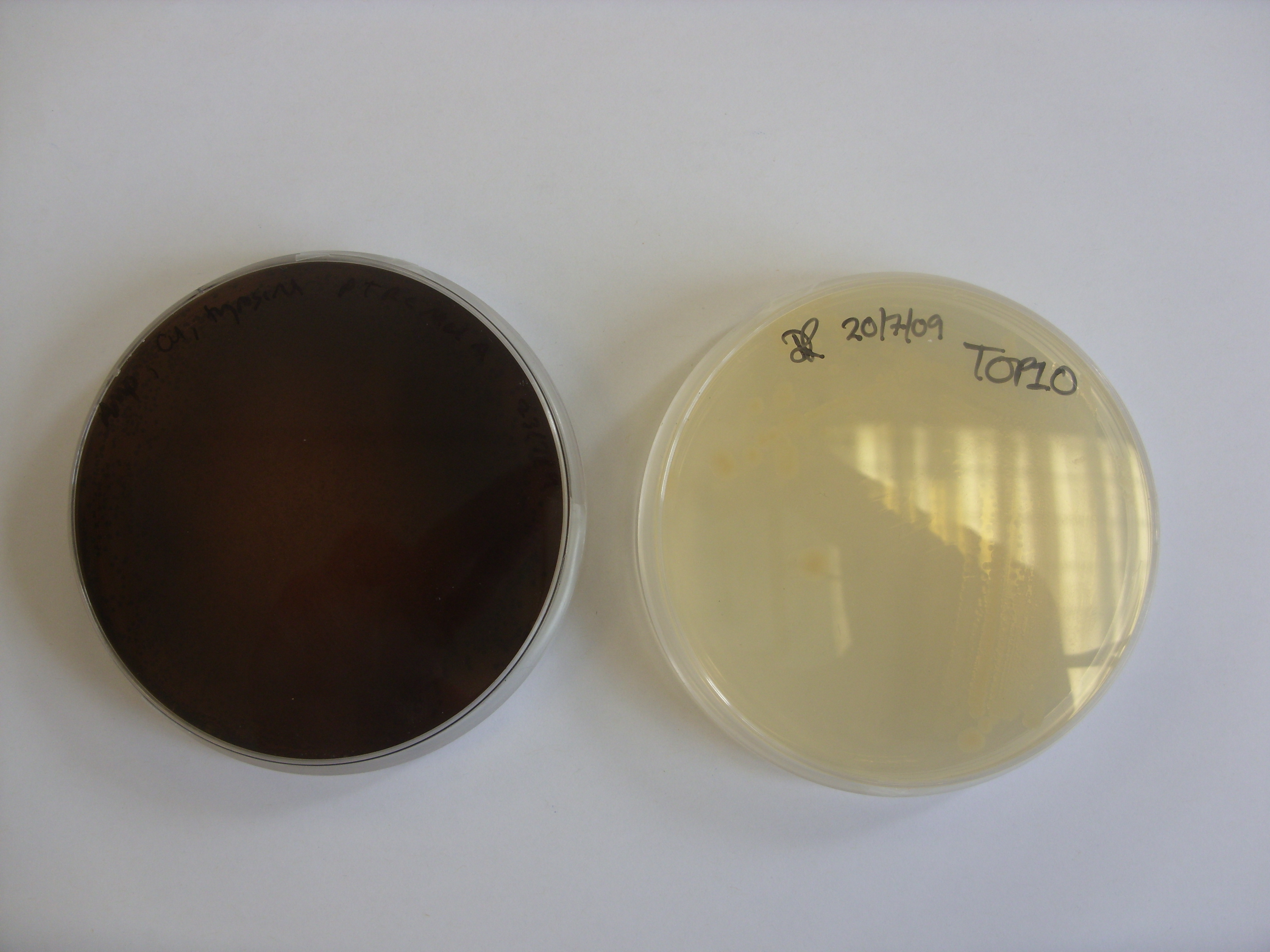
We transformed Top10 E. coli with pTRCmelA and plated them, using LA supplemented with 15ug/ml copper, 0.2ug/ul tyrosine. Below is a plate that was incubated at 37 degrees for 24 hours and then left on the bench at room temperature over the weekend. Pigment was clearly produced, and it appears to have diffused out of the colonies.
Left: Top10 transformed with plasmid containing the MelA gene. Right: untransformed Top10.
Control Experiments
To show that the brown colour was a result of the MelA gene and not natural oxidation of the LA supplements, a control plate without any bacteria was incubated for the same amount of time and showed no change in colour (data not shown).
Optimization
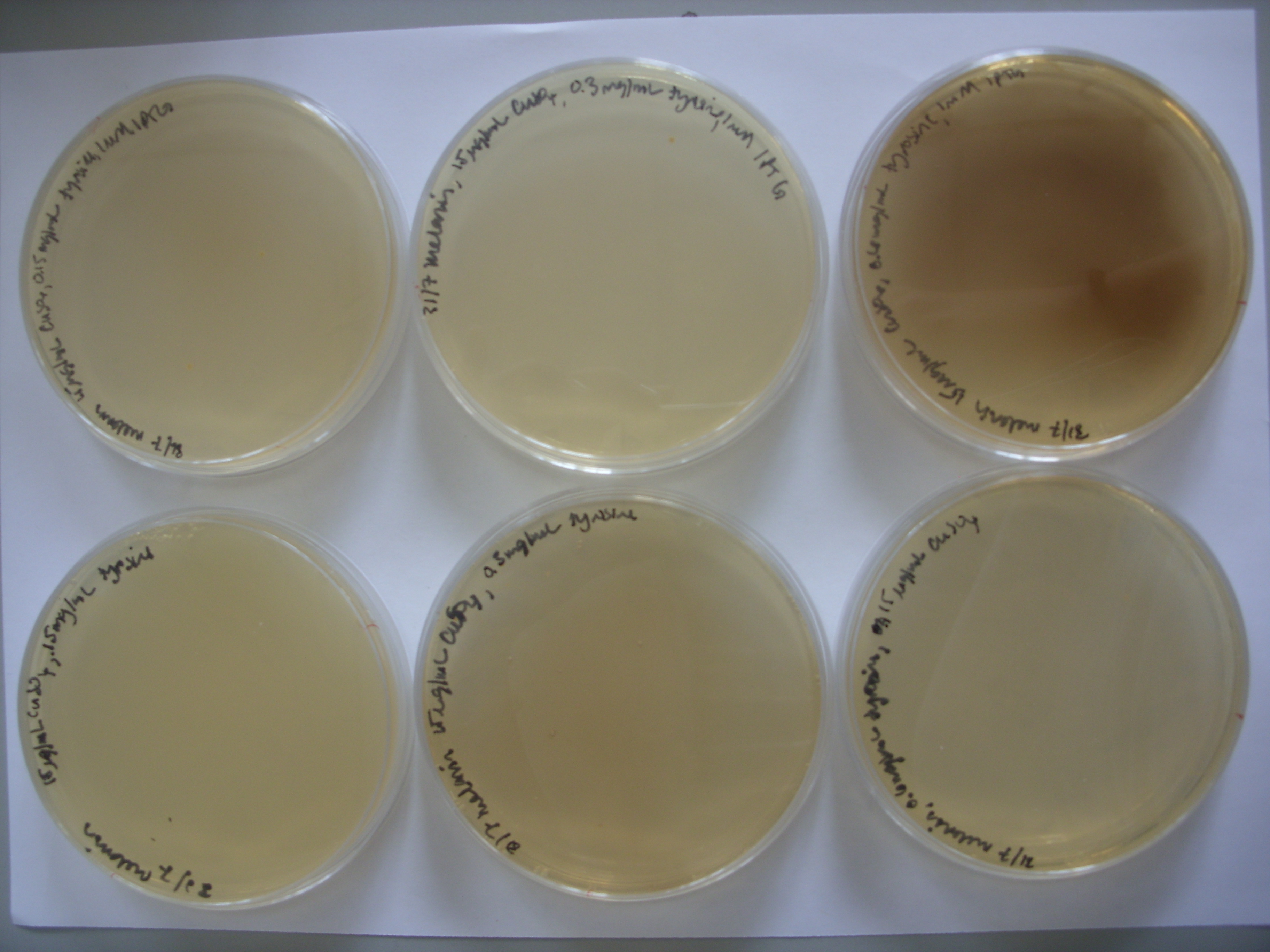
On pTRCmelA, the MelA gene is under the control of the lac repressor. The photo above shows leaky expression of the promoter, as no IPTG was not added. We then experimented with the addition of IPTG and varying tyrosine concentrations to see the effect on pigment production. As the photo below shows, the greatest pigment production was achieved with IPTG induction and the highest concentration of tyrosine.
From left to right, top row: 1mM IPTG with 0.075 mg/mL tyrosine, 1mM IPTG and 0.3 mg/mL tyrosine, and then 1mM IPTG and 0.6 mg/mL tyrosine From left to right, bottom row: 0.075 mg/mL tyrosine, 0.3 mg/mL tyrosine, and then 0.6 mg/mL tyrosine.
 "
"