Team:Cornell/Project/Background
From 2009.igem.org
(→Cadmium Contamination) |
|||
| Line 15: | Line 15: | ||
=Background= | =Background= | ||
==Cadmium Contamination== | ==Cadmium Contamination== | ||
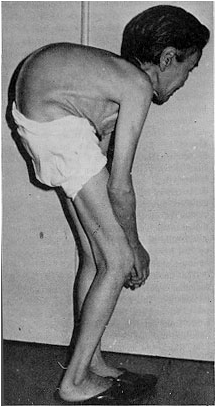
| - | Cadmium (Cd) is a toxic heavy metal which has no known biological function. Ingestion of Cd-contaminated water can induce diarrhea, severe vomiting, bone fracture (Itai-Itai disease), infertility, damage to the central nervous and immune systems, cancer development and ultimately death. Once affected by | + | Cadmium (Cd) is a toxic heavy metal which has no known biological function. Ingestion of Cd-contaminated water can induce diarrhea, severe vomiting, bone fracture (Itai-Itai disease), infertility, damage to the central nervous and immune systems, cancer development and ultimately death. Once affected by cadmium, there is no effective treatment for humans (Agency for Toxic Substances and Disease Registry). For instance, in late 20th century Japan, cadmium contamination of water in the Jingzu River led to significant kidney problems and Itai-Itai bone disease in a fairly large population. In another study, Cd concentration was directly related to mortality rate.[1] |
| + | [[Image:Itai_itai.png|right|frame|Patient suffering from prolonged exposure to cadmium]] | ||
| + | Of late, cadmium levels have been better controlled by industrial regulation, but it still poses formidable problems. The Environmental Protection Agency (EPA) has set cadmium level regulations at a maximum of 5µg/L in drinking water. Of the 1,669 hazardous waste sites on the EPA's National Priorities List (NPL), 1,014 contain cadmium. The Australian National Pollutant Inventory (NPI) has ranked Cd number 6 out of 400 of the most toxic substances based on health and environmental hazards and human and environmental exposure to the substance. According to the NPI, Cd has a total hazard rating of 4.3 as compared to 2.8 for Carbon Monoxide and 4.0 for Arsenic. | ||
| - | + | Major sources of Cd contamination include fertilizers, sewage sludge, manure and atmospheric deposition. The amount of Cd released into the environment from human activities has actually been about 10 times greater than the amount predicted from natural sources.[2] Likewise, the amount of Cd released into the agricultural environment has also increased significantly over the last century.[3] In fact, Cd-contaminated sewage is often used for irrigation purposes in many parts of the world, especially in developing nations.[2] Crops grown in these Cd-contaminated soils are then sold in markets without any detoxification treatment. As of now, consuming Cd-contaminated crops is one of the greatest cadmium-related problems for humans. In a study conducted in Faisalabad in Pakistan, it was found that untreated sewage used for irrigation had contamination levels that were three times the allowed level (0.03 mg.L−1 as compared to 0.01 mg.L−1) for irrigation water. Furthermore, the study found that consuming crops grown in these Cd-contaminated soils for extended periods of time can cause high levels of Cd to accumulate in humans which would lead to a number of illnesses.[2] Since similar irrigation methods are used in a number of other developing countries, it would be extremely useful to have a simple method of measuring cadmium levels in water before using it for mass irrigation purposes. | |
| - | + | ||
| - | Major sources of Cd contamination include fertilizers, sewage sludge, manure and atmospheric deposition. The amount of Cd released into the environment from human activities has actually been about 10 times greater than the amount predicted from natural sources.[2] Likewise, the amount of Cd released into the agricultural environment has also increased significantly over the last century.[3] In fact, Cd-contaminated sewage is often used for irrigation purposes in many parts of the world, especially in developing nations.[2] Crops grown in these Cd-contaminated soils are then sold in markets without any detoxification treatment. As of now, consuming Cd-contaminated crops is one of the greatest | + | |
Recently developed cadmium detection methods include transmission-based localized surface plasmon resonance (LSPR) fiber-optic probes[4], prompt gamma-ray neutron activation analysis (PGNAA) [5], and a solid surface fluorescence based flow-through optosensor[6] have been developed to determine cadmium ion concentration, but the cost efficiencies of such instruments significantly exceed the cost of a simple bacterial biosensor. | Recently developed cadmium detection methods include transmission-based localized surface plasmon resonance (LSPR) fiber-optic probes[4], prompt gamma-ray neutron activation analysis (PGNAA) [5], and a solid surface fluorescence based flow-through optosensor[6] have been developed to determine cadmium ion concentration, but the cost efficiencies of such instruments significantly exceed the cost of a simple bacterial biosensor. | ||
Revision as of 02:36, 22 October 2009
BackgroundCadmium ContaminationCadmium (Cd) is a toxic heavy metal which has no known biological function. Ingestion of Cd-contaminated water can induce diarrhea, severe vomiting, bone fracture (Itai-Itai disease), infertility, damage to the central nervous and immune systems, cancer development and ultimately death. Once affected by cadmium, there is no effective treatment for humans (Agency for Toxic Substances and Disease Registry). For instance, in late 20th century Japan, cadmium contamination of water in the Jingzu River led to significant kidney problems and Itai-Itai bone disease in a fairly large population. In another study, Cd concentration was directly related to mortality rate.[1] Of late, cadmium levels have been better controlled by industrial regulation, but it still poses formidable problems. The Environmental Protection Agency (EPA) has set cadmium level regulations at a maximum of 5µg/L in drinking water. Of the 1,669 hazardous waste sites on the EPA's National Priorities List (NPL), 1,014 contain cadmium. The Australian National Pollutant Inventory (NPI) has ranked Cd number 6 out of 400 of the most toxic substances based on health and environmental hazards and human and environmental exposure to the substance. According to the NPI, Cd has a total hazard rating of 4.3 as compared to 2.8 for Carbon Monoxide and 4.0 for Arsenic. Major sources of Cd contamination include fertilizers, sewage sludge, manure and atmospheric deposition. The amount of Cd released into the environment from human activities has actually been about 10 times greater than the amount predicted from natural sources.[2] Likewise, the amount of Cd released into the agricultural environment has also increased significantly over the last century.[3] In fact, Cd-contaminated sewage is often used for irrigation purposes in many parts of the world, especially in developing nations.[2] Crops grown in these Cd-contaminated soils are then sold in markets without any detoxification treatment. As of now, consuming Cd-contaminated crops is one of the greatest cadmium-related problems for humans. In a study conducted in Faisalabad in Pakistan, it was found that untreated sewage used for irrigation had contamination levels that were three times the allowed level (0.03 mg.L−1 as compared to 0.01 mg.L−1) for irrigation water. Furthermore, the study found that consuming crops grown in these Cd-contaminated soils for extended periods of time can cause high levels of Cd to accumulate in humans which would lead to a number of illnesses.[2] Since similar irrigation methods are used in a number of other developing countries, it would be extremely useful to have a simple method of measuring cadmium levels in water before using it for mass irrigation purposes. Recently developed cadmium detection methods include transmission-based localized surface plasmon resonance (LSPR) fiber-optic probes[4], prompt gamma-ray neutron activation analysis (PGNAA) [5], and a solid surface fluorescence based flow-through optosensor[6] have been developed to determine cadmium ion concentration, but the cost efficiencies of such instruments significantly exceed the cost of a simple bacterial biosensor. References[1]T. Ishihara, E. Kobayashi, Y. Okubo, Y. Suwazono, T. Kido, M. Nishijyo, H. Nakagawa and K. Nogawa (2001), Association between cadmium concentration in rice and mortality in the Jinzu river basin, Japan, Toxicology 163, p.23-28 [2]Qadir, A. Ghafoor and G. Murtaza (2000), Cadmium Concentration in Vegetables Grown on Urban Soils Irrigated with Untreated Municipal Sewage, Environment, Development and Sustainaility 2(1), p.13-21. [3]Jones, K.S., Jackson, A. and Johnston, A.E (1992), Evidence for an increase in the Cd content of herbage since the 1860s, Environ. Sci. Technol. 26, p.834-836 [4]Lin, Tsao-Jen, and Mon-Fu Chung (2009), Detection of Cadmium by a Fiber-Optic Biosensor Based on Localized Surface Plasmon Resonance, Biosensors and Bioelectronics 24(5), p.1213-8. [5]Grazman, B.L., and Schweikert, E.A (2005). A brief review of the determination of cadmium by prompt gamma-ray neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry 152(2), p.497 – 506 [6]Garcia-Reyes et al. Sensing of trace amounts of cadmium in drinking water using a single fluorescence-based optosensor. Microchemical Journal 82(1), p.94-99 |
 "
"