Team:DTU Denmark/genetic design
From 2009.igem.org
| Line 170: | Line 170: | ||
</font> | </font> | ||
<font color="" face="arial" size="2"> | <font color="" face="arial" size="2"> | ||
| - | <a href="https://2009.igem.org/Team:DTU_Denmark/ | + | <a href="https://2009.igem.org/Team:DTU_Denmark/genetic_design" CLASS=leftbar>- Genetic design</a><br> |
| - | <a href="https://2009.igem.org/Team:DTU_Denmark/ | + | <a href="https://2009.igem.org/Team:DTU_Denmark/applications" CLASS=leftbar>- Applications and perspectives</a><br> |
| - | <a href="https://2009.igem.org/Team:DTU_Denmark/ | + | <a href="https://2009.igem.org/Team:DTU_Denmark/results" CLASS=leftbar>- Results</a><br> |
| + | <a href="https://2009.igem.org/Team:DTU_Denmark/safety" CLASS=leftbar>- Safety considerations</a><br><br> | ||
</font> | </font> | ||
<font color="#990000" face="arial" size="3"> | <font color="#990000" face="arial" size="3"> | ||
| - | <br>The USER | + | <br>The USER assembly standard<br><br> |
</font> | </font> | ||
<font face="arial" size="2"> | <font face="arial" size="2"> | ||
| - | <a href="https://2009.igem.org/Team:DTU_Denmark/USERprinciple" CLASS=leftbar>- | + | <a href="https://2009.igem.org/Team:DTU_Denmark/USERprinciple" CLASS=leftbar>- USER fusion of biobricks</a><br><br> |
| - | + | ||
| - | + | ||
</font> | </font> | ||
<font color="#990000" face="arial" size="3"> | <font color="#990000" face="arial" size="3"> | ||
| - | <br>USER | + | <br>USER fusion primer design software<br><br> |
</font> | </font> | ||
<font face="arial" size="2"> | <font face="arial" size="2"> | ||
| Line 234: | Line 233: | ||
<p><b>Modelling</b><br> | <p><b>Modelling</b><br> | ||
| - | To guide the design process and to simulate how our genetic device would operate in vivo, we performed thorough mathematical modelling of the relation between the input and output of our system (the detailed model is available <a href="http://partsregistry.org/Part:BBa_K194003" target="_blank">here</a>). The model predicts how the GFP-concentration in a transformed cell relates to the internal NAD+/NADH level (indicative of the redox state).<br> | + | To guide the design process, and to simulate how our genetic device would operate in vivo, we performed thorough mathematical modelling of the relation between the input and output of our system (the detailed model is available <a href="http://partsregistry.org/Part:BBa_K194003" target="_blank">here</a>). The model predicts how the GFP-concentration in a transformed cell relates to the internal NAD+/NADH level (indicative of the redox state).<br> |
| - | The model takes the following into account: | + | The model takes the following into account: |
| - | + | ||
i) transcription factor activation,<br> | i) transcription factor activation,<br> | ||
ii) promoter activation, <br> | ii) promoter activation, <br> | ||
| Line 242: | Line 241: | ||
iv) synthesis, dilution and degradation of GFP. <br><br> | iv) synthesis, dilution and degradation of GFP. <br><br> | ||
| - | + | transcription factor activation, promoter activation, synthesis and degradation of mRNA and finally synthesis, dilution and degradation of GFP. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | This model formed the basis of the genetic design of the The redoxilator. Importantly it provided understanding of the influence of each of the parameters when designing the system, e.g. that in order to achieve fast response time a fast degradable GFP was needed as reporter. However, since many parameters specific for this system are not yet available, the model is not expected to make exact predictions but to ease the understanding of the design "principles" | ||
</p> | </p> | ||
| Line 319: | Line 314: | ||
<strong>References</strong><br> | <strong>References</strong><br> | ||
[Brekasis and Paget, 2003] Brekasis, D. and Paget, M. S. B. (2003). A novel sensor of nadh/nad+ redox poise in streptomyces coelicolor a3(2). EMBO J, 22(18):4856–4865.<br> | [Brekasis and Paget, 2003] Brekasis, D. and Paget, M. S. B. (2003). A novel sensor of nadh/nad+ redox poise in streptomyces coelicolor a3(2). EMBO J, 22(18):4856–4865.<br> | ||
| - | |||
| - | |||
| - | |||
[Sadowski et al., 1988] Sadowski, I., Ma, J., Triezenberg, S., and Ptashne, M. (1988). Gal4-vp16 is an unusually potent transcriptional activator. Nature, 335(6190):563–564. | [Sadowski et al., 1988] Sadowski, I., Ma, J., Triezenberg, S., and Ptashne, M. (1988). Gal4-vp16 is an unusually potent transcriptional activator. Nature, 335(6190):563–564. | ||
</p> | </p> | ||
| Line 333: | Line 325: | ||
<td width="182px" height="100%" valign="top" bgcolor=DDDDDD class="greybox"> | <td width="182px" height="100%" valign="top" bgcolor=DDDDDD class="greybox"> | ||
<font face="arial" size="2"> | <font face="arial" size="2"> | ||
| - | <b> | + | <b>Why yeast?</b><br><br> |
| Line 339: | Line 331: | ||
| - | + | <p>The utilization of improved micro-organisms for industrial processes is a fact for centuries. From the early stages in the preparation of fermented food and beverages until nowadays. Recent advances in biochemistry, engineering and genetic manipulation techniques, led scientist and engineers to improve micro-organisms in order to enhance their metabolic capabilities for biotechnological applications. Along with these improvements, a far more rational and direct approach to strain improvement have been employed, of what we call Metabolic Engineering. What distinguishes Metabolic Engineering from the classical approaches is the application of advanced analytical tools for identification of suited targets for genetic modifications or even the use of mathematical models to perform in silico design of optimized micro-organisms. The consequences of the changes introduced in these engineered strains can then suggest further modifications to improve cellular performance and therefore Metabolic Engineering can be seen as a cyclic process made of continuous iterations between experimental and analytical work.<br><br> | |
| - | + | Among all possible micro-organisms, <i>Saccharomyces cerevisiae</i> is a very well-suited candidate since it is recognized as being GRAS (“generally regarded as safe”).<br><br> | |
| - | + | Due to its long history of application in the production of consumable products such as ethanol and baker’s yeast, <i>Saccharomyces cerevisiae</i> has a very well-established fermentation and process technology for large-scale production. The availability of its complete genome sequence of in 1996, and the numerous possibilities for genetic modifications by recombinant DNA technology that came with that, made of yeast a perfect model organism within the field of biotechnology.</p> | |
| - | + | ||
| - | + | ||
Revision as of 22:28, 20 October 2009

| Home | The Team | The Project | Parts submitted | Modelling | Notebook |
|
The redoxilator - Genetic design - Applications and perspectives - Results - Safety considerations The USER assembly standard - USER fusion of biobricks USER fusion primer design software - Abstract - Instructions - Output format |
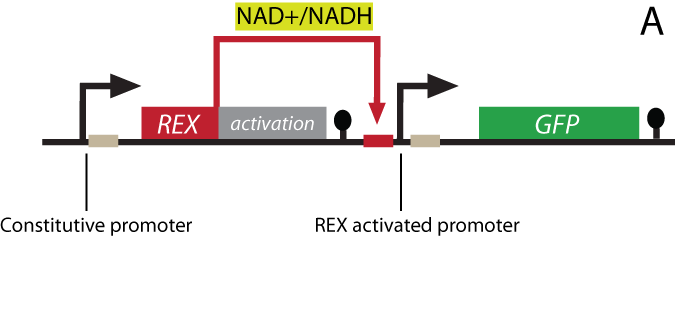
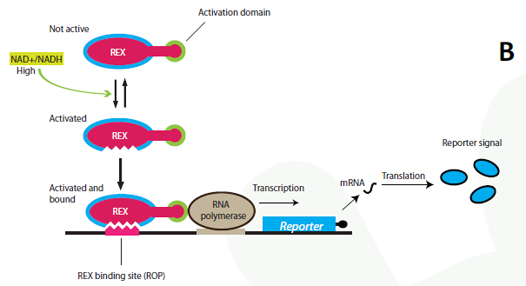
The project Genetic design We have designed and physically constructed a genetic system that couples the intracellular NAD+/NADH level to the gene expression of a reporter protein. The system has potentially many applications including in vivo online monitoring of the redox poise, production optimization and cancer research with yeast as a model organism (see Applications). The NAD+/NADH ratio is sensed by a system originating in Streptomyces coellicolor. In S. coellicolor the protein REX is a repressor and controls the gene expression of multiple genes by recognizing and binding to a specific DNA-sequence termed ROP (Rex operator). NAD+ and NADH compete to associate with Rex, but only a REX:NAD+ association can bind the ROP DNA-sequence (Brekasis and Paget, 2003). In S. coellicolor REX DNA binding represses expression of target genes, by physically hindering RNA-polymerases from binding the promoter. As the transcription machinery of eukaryotes is different and more complicated, there are no guarantee that repression will be effective in eukaryotes. REX has therefore been fused to a eukaryotic transcriptional activator, a widely used technique applied for the investigation of the GAL proteins and other systems (Sadowski et al. 1988). The REX-activator fusion-protein is able to bind the ROB sequence placed upstream of a minimal eukaryotic promoter that only supports transcription upon activation. A certain NAD+/NADH ratio will activate the Redoxilator to recognize the ROB promoter, resulting in transcription of the reporter gene.
Gene design and redox regulation Design specifications
The genetic elements and the requirements they need to fulfill are listed in the following table. The detailed description of the used genetic elements will not be made publicly available due to IP rights. The elements have been selected solely on their properties, and are from a variety of organisms - several of them are biobricks. All elements are codon optimized for S. cerevisiae
Modelling Description and requirements of the genetic elements
References |
Why yeast? The utilization of improved micro-organisms for industrial processes is a fact for centuries. From the early stages in the preparation of fermented food and beverages until nowadays. Recent advances in biochemistry, engineering and genetic manipulation techniques, led scientist and engineers to improve micro-organisms in order to enhance their metabolic capabilities for biotechnological applications. Along with these improvements, a far more rational and direct approach to strain improvement have been employed, of what we call Metabolic Engineering. What distinguishes Metabolic Engineering from the classical approaches is the application of advanced analytical tools for identification of suited targets for genetic modifications or even the use of mathematical models to perform in silico design of optimized micro-organisms. The consequences of the changes introduced in these engineered strains can then suggest further modifications to improve cellular performance and therefore Metabolic Engineering can be seen as a cyclic process made of continuous iterations between experimental and analytical work. |
| Comments or questions to the team? Please Email us -- Comments of questions to webmaster? Please Email us |
 "
"