Team:EPF-Lausanne/Notebook/Wet Lab
From 2009.igem.org
Contents |
Wet Lab
July
06.07.09
LOVTAP plasmid AND TrpR plasmid were transformed in competent E. Coli following received protocol, and grown overnight (see Lab book for more details).
One problem: we actually don't know TrpR plasmid resistance, so we tried with three resistances available in the lab: Amp., Kana. and Chl.
LOVTAP is in a plasmid called pCal-n (see picture below):
Some comments on the plasmid:
-CBP is a small peptide with which we could purify LOVTAP protein
-Thrombin target is a nucleotidic sequence that can be recognized by thrombin a peptidase. This peptidase will cut CBP once LOVTAP is purified
07.07.09
We have to grow the 3 strains generously sent by Tom Beatty
The three strains are :
- R.Palustris CEA001 (wild type) ; should be grown on LB medium only
- R.Palustris BPHP1+ ; should be grown on LB with gentamycin (100 micrograms/ml)
- E.Coli DH10B (pBPH/hmu0) ; should be grown on LB with gentamycin (20 micorgrams/ml)
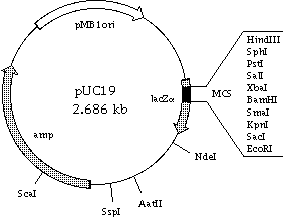
The transformed LOVTAP and TrpR worked well (N.B. the plasmid of TrpR is pUC19 so the antibiotic resistance is Amp -> see below)
We did the glycerol stock, located in the -80 fridge, first floor of the iGEM compartement.
Then, a miniprep was done with both cultures. A LOVTAP plasmid aliquot was done, a TrpR plasmid aliquot was done, located in the -20 fridge, 2nd floor.
08.07.09
1. R. Palustris culture grew. A glycerol stock has been done. A pellet is on the fridge level 2, waiting for a miniprep.
2. iGEM parts have been transformed:
| Part | Characteristic | Resistance | Well (Kit Plate) |
|---|---|---|---|
|
[http://partsregistry.org/Part:BBa_B0010 BBa_B0010] |
Terminator |
A |
13D (1) |
|
[http://partsregistry.org/Part:BBa_R0010 BBa_R0010] |
Promoter LacI |
A |
1D (1) |
|
[http://partsregistry.org/Part:BBa_B0030 BBa_B0030] |
RBS |
A |
1H (1) |
|
[http://partsregistry.org/Part:BBa_E0240 BBa_E0240] |
RBS-GFP-TER |
A |
12M (1) |
|
[http://partsregistry.org/Part:BBa_I13507 BBa_I13507] |
RBS-mRFP-TER |
A |
22O (1) |
|
[http://partsregistry.org/Part:BBa_J13002 BBa_J13002] |
pTetR-RBS |
A |
13B (1) |
09.07.09
1. Miniprep and isolations of the yesterday transformed plasmids. (cf. 08.09.09 subpart)
Concentrations of the plasmids: cf. lab notebook pp. 8-9
2. PCR of pCal-n to PCR up LOVTAP with the different primers designed the 06.09.09, the products are:
- Prom_T7-RBS-CBP-LOVTAP
- RBS-CBP-LOVTAP
- CBP-LOVTAP
- LOVTAP
Result: Prom_T7-RBS-CBP-LOVTAP didn't worked, the other were ok.
3. An agarose gel was runned to check PCR products
4. PCR products were digested with EcorI and SpeI and [http://partsregistry.org/Part:BBa_B0010 BBa_B0010] (plasmid chosen containing the terminator) was digested with EcorI and XbaI, the digestion products were treated with phosphatase. Then, PCR products were purified.
Finally, LOVTAP (PCR products) were ligated on [http://partsregistry.org/Part:BBa_B0010 BBa_B0010] (plasmid chosen containing the terminator).
5. Two more iGEM parts have been transformed:
| Part | Characteristic | Resistance | Well (Kit Plate) |
|---|---|---|---|
|
[http://partsregistry.org/Part:BBa_I6007 BBa_I6007] |
Double repressor: called Inverter TetR |
A |
1C (2) |
|
[http://partsregistry.org/Part:BBa_P1010 BBa_P1010] |
Death Cassette |
C |
5E (1) |
Remarks: [http://partsregistry.org/Part:BBa_P1010 BBa_P1010], the death gene has to be transformed in ccdB (death gene) resistant cells: One Shot ccdB survival 2T1 E. Coli
10.07.09
Miniprep of the yesterday transformations were done, glycerol stock of [http://partsregistry.org/Part:BBa_I6007 Inverter TetR (BBa_I6007)] and [http://partsregistry.org/Part:BBa_P1010 Death Cassette (BBa_P1010)] were done and finally they were put at -80°C.
As Prom_T7-RBS-CBP-LOVTAP PCR product migration on the gel didn't worked (in fact there wasn't enough products) July the 9th, a second PCR was done with more cycles (40) to check wether the the primers were accurately designed.
Results: The primers are correct -> Prom_T7-RBS-CBP-LOVTAP was correctly amplified.
A digestion-ligation according iGEM special protocol Biobrick_Assembly_Manual was done with LacI and RBS. And Term was digested (with E and X) to linearize the plasmid.
As [http://partsregistry.org/Part:BBa_I6007 Inverter TetR (BBa_I6007)] and [http://partsregistry.org/Part:BBa_P1010 Death Gene (BBa_P1010)] were transformed with a contaminated SOC, we did a PCR with the isolated plasmids to check if the plasmids were correct.
Results: [http://partsregistry.org/Part:BBa_P1010 Death Cassette (BBa_P1010)] sample contain the correct plasmid, [http://partsregistry.org/Part:BBa_I6007 Inverter TetR (BBa_I6007)] doesn't.
Finally, a gel extraction was done to purify the digested [http://partsregistry.org/Part:BBa_B0010 Terminator (BBa_B0010)] (linearized plasmid)
13.07.09
[http://partsregistry.org/Part:BBa_I6007 Inverter TetR (BBa_I6007)] was transformed again. And the new plasmid (created on July the 10th, 10.07.09) LacI-RBS was transformed on DH5-alpha competent cells.
We failed in purifing our [http://partsregistry.org/Part:BBa_B0010 Terminator (BBa_B0010)], do it again tomorrow, beginning with the digestion, etc.
14.07.09
The digestion of the [http://partsregistry.org/Part:BBa_B0010 Terminator (BBa_B0010)] was done, in order to purify it once more, using gel extraction.
Miniprep of LacI-RBS and Inverter TetR, and PCR of the two of them.
The linearized [http://partsregistry.org/Part:BBa_B0010 Terminator (BBa_B0010)] was runned on an agarose gel and purified with a gel extraction kit.
Even though linearized [http://partsregistry.org/Part:BBa_B0010 Terminator (BBa_B0010)] concentration was very low, we tried to ligate the previously amplified LOVTAP (08.07.09) in linearized [http://partsregistry.org/Part:BBa_B0010 Terminator (BBa_B0010)]
15.07.09
Miniprep of LacI-RBS (again, because we had a very low concentration yesterday). We obtained a better concentration. Transformation of LovTAP-Term.
 "
"