Team:EPF-Lausanne/Results/EDS
From 2009.igem.org
(→Evolution of Pression, Volume and Temperature) |
(→CYS450 - FMN) |
||
| Line 133: | Line 133: | ||
The real ratio was calculated as follow: the threshold for the sulfur atom to be in the IN conformation is 4 (or less) ängström from the C4A carbon atom of the FMN. | The real ratio was calculated as follow: the threshold for the sulfur atom to be in the IN conformation is 4 (or less) ängström from the C4A carbon atom of the FMN. | ||
| - | So the actual ratios were IN = 0. | + | So the actual ratios were IN = 0.2979 => 29.79% and OUT = 0.7021 => 70.21% |
It is very interesting that these resulting ratios are exactly the same as the ones found by [http://www.ncbi.nlm.nih.gov/pubmed/18001137 Halavaty et al.] in vitro. | It is very interesting that these resulting ratios are exactly the same as the ones found by [http://www.ncbi.nlm.nih.gov/pubmed/18001137 Halavaty et al.] in vitro. | ||
Revision as of 16:15, 21 October 2009
Contents |




We run an equilibration of 80ns using 32 processors on the dark state (2v0u). It took a month of computation to have this result.
Here is a movie over the trajectory file.
Validation of dark state simulation
Here we look at the output to check input parameters.
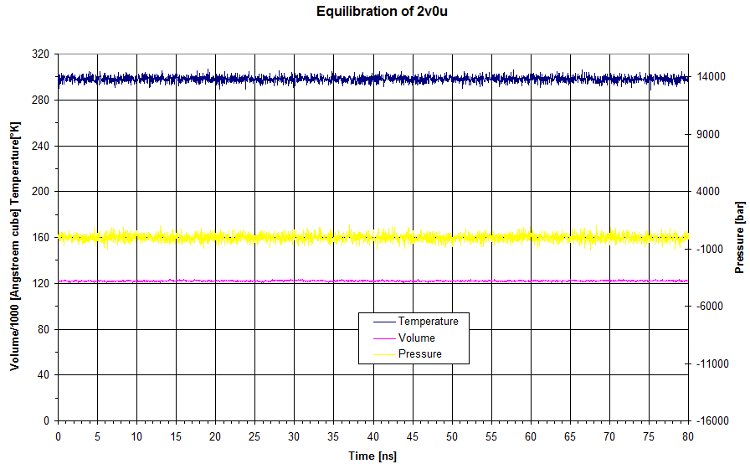
Evolution of Pression, Volume and Temperature
The raw data for the equilibration match what we set for the NPT. Pressure and temperature are kept constant using NAMD dynamic. The volume is quite constant as well.
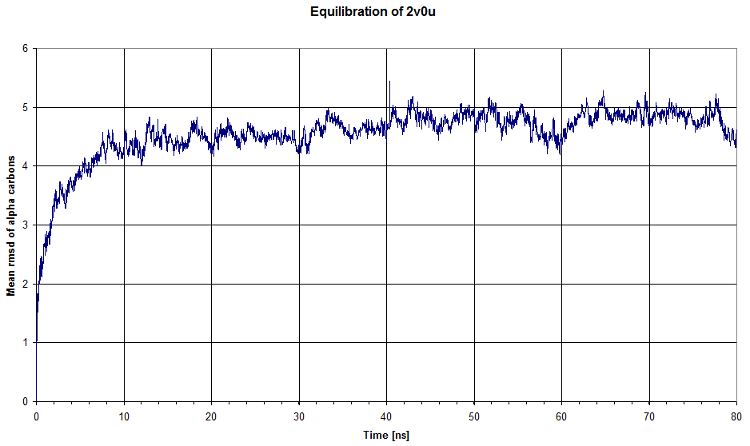
RMSD
Then we computed the evolution of the rmsd compared to the first timestep of equilibration. We see that there is a plateau after ~40ns, which means that our system's energy is reaching a minimum. That's clearly what we expected.
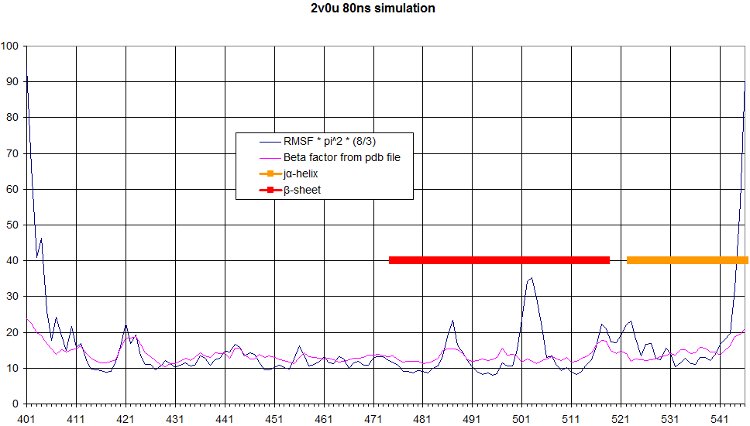
RMSF
The comparison of the RMSF to the beta factor measured during crystallography of all alpha carbons over the simulation is a nice validation of our simulation. We get quite similar curves, with some differences at one end of the protein. We see in the movie that this part moves a lot.
Here we computed the oscillation of RMSF in function of residue number, and we highlight the interesting part of our protein, namely the beta sheet and the alpha helix.
Analysis of the simulation
We have organized our analysis on 2 main ideas:
- Find a structural change in the Jα helix based on the simulation using namd.
- Find residues showing different comportment in dark and light state
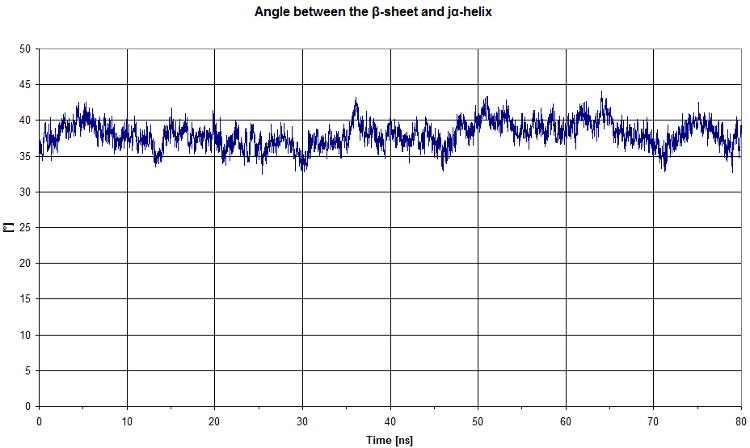
Angle between beta sheet and alpha helix
First, we start by looking at the angle between the beta sheet and the Jα helix.
Click here to see the code used in VMD to get angle data
We get a quite constant value. It would be more interesting to compare this graph to the light state, however in both states there is no clear movement.
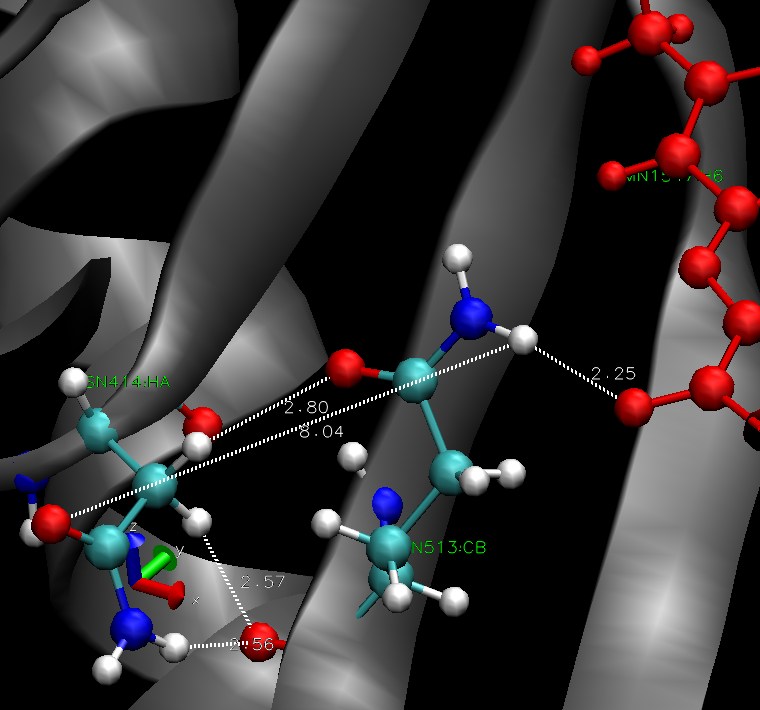
GLN513 - ASN414 - FMN
The Jα helix is stabilized by h-bonds to the beta sheet. These bonds are supposed to be disrupted by the conformational change in the dark state. The residues 513 seems to be involved in stabilisation of FMN through hydrogen bonds. We hope it is linked to beta sheet, more precisely the residue 414. There is a picture of the situation, residue ASN 414 is on the left (beta sheet), GLN513 in the middle and the FMN is in red. All the hydrogen bonds we investigated over the simulation are pictured.
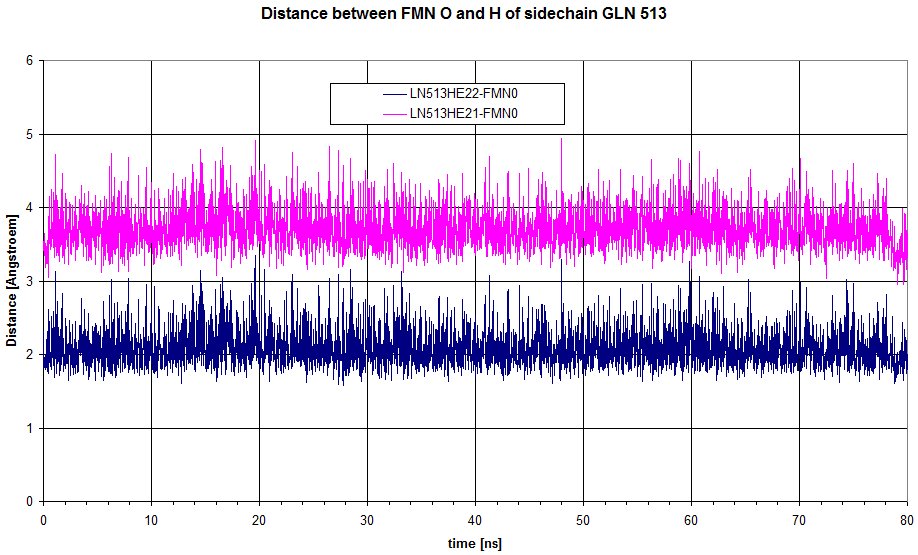
Here is a plot of the distance between the 2 hydrogens from sidechain of GLN513 to the oxygen of FMN. HE22 is definitely involved in an hydrogen bond, but doesn't move enough to loose the interaction.
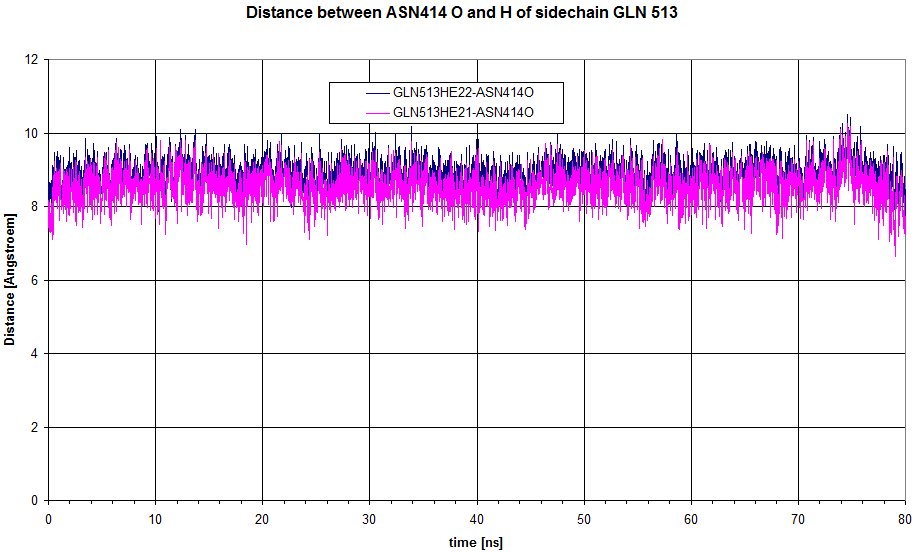
We can have a look at the distance between O of sidechain of ASN 414 to Hs of GLN513.
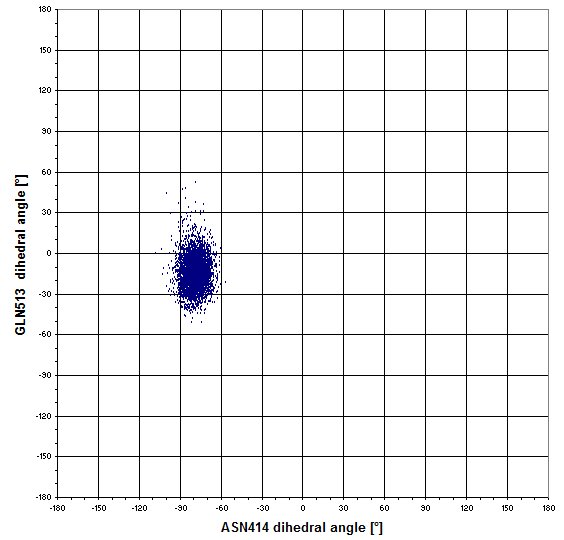
We imagined that the sidechains of GLN513 could move in regard to the position of ASN414, that's why we wanted to check it with the following graph, which plot the dihedral angles of Asn 414 against the dihedral angles of Gln513.
Finally we concluded that it was a wrong idea because there is a single combination on the graph.
CYS450 - FMN
An interesting residue to study in the dark state is the residue n° 450, which is the cystein that reacts with the cofactor. In fact, this is the residue that initiates the general movement for the conformational change of the protein. For this reason, this analysis is crucial to understand the beginning of the conformational change. Let's start with a picture of the situation, where you can see the distance between CYS450 and FMN:
Here we plot the distance between the sulfur atom of the cystein and the FMN carbon which is attacked by the sulfur atom upon light activation:
We can define two conformation, depending whether or not the sulfur atom is pointing toward the cofactor:
- in the IN conformation, the sulfur point toward FMN. It happens during 30% of the time
- in the OUT conformation, it doesn't point toward the cofactor, during the restant 70% of the time
These percentages were previously discovered by an analysis of [http://www.ncbi.nlm.nih.gov/pubmed/18001137 Halavaty et al.] in vitro.
Here we can confirm that the sulfur atom is 70% of the time in the OUT conformation (doesn't point toward the cofactor, FMN). In the plot, it is represented by the fact that the sulfur atom is far from the C4A carbon of the FMN (the one which is attacked by the sulfur atom upon light activation).
Also our plot confirm that the sulfur atom is 30 % of the time in the IN conformation (point toward the cofactor, FMN). In the plot, it is represented by the fact that the sulfur atom is near the C4A carbon of the FMN.
The real ratio was calculated as follow: the threshold for the sulfur atom to be in the IN conformation is 4 (or less) ängström from the C4A carbon atom of the FMN.
So the actual ratios were IN = 0.2979 => 29.79% and OUT = 0.7021 => 70.21%
It is very interesting that these resulting ratios are exactly the same as the ones found by [http://www.ncbi.nlm.nih.gov/pubmed/18001137 Halavaty et al.] in vitro.
| IN conformation | OUT conformation |
|---|---|
Here is the plot of the dihedral angle of this residue to see how many time the cystein point toward the FMN:
This plot show exactly the same conformational ratio (70% OUT 30% IN) than the distance plot.
Some useful distances
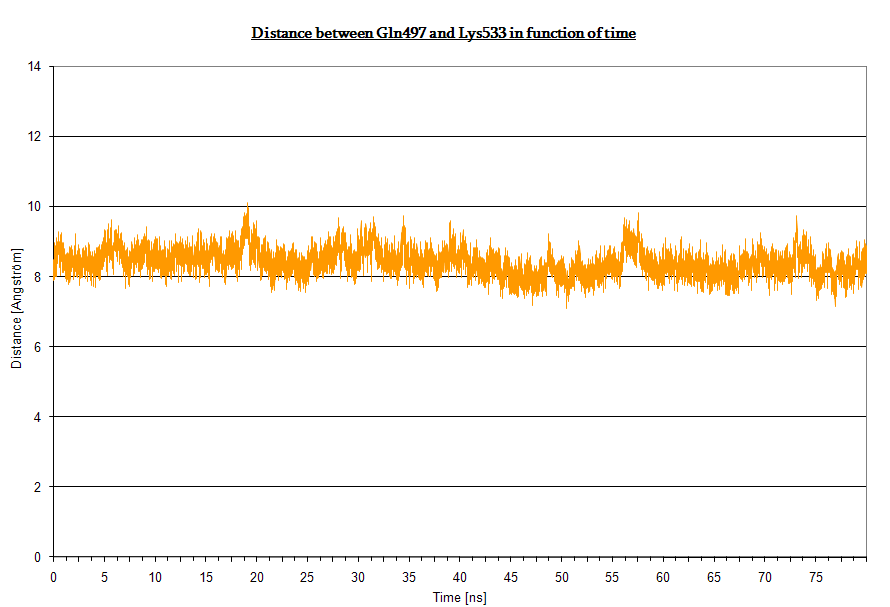
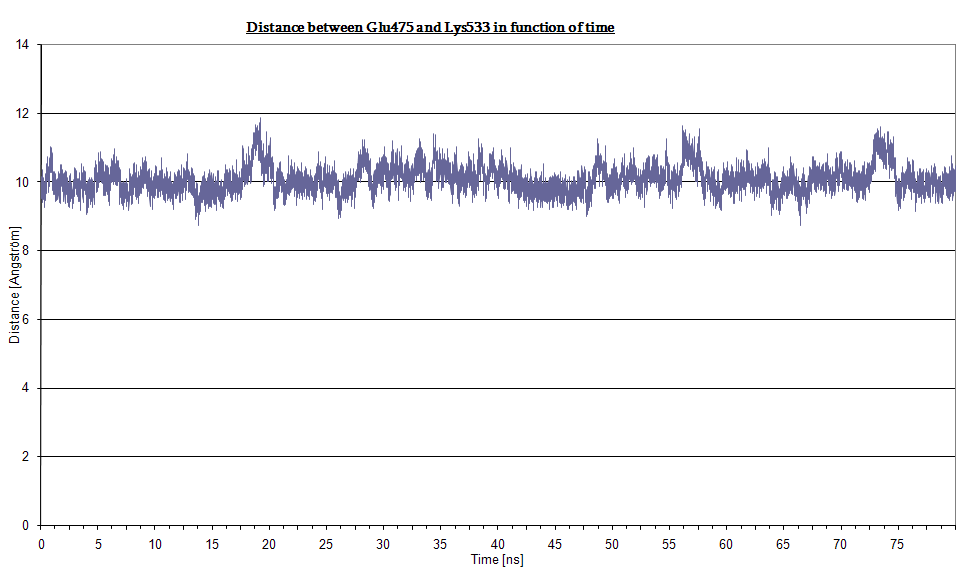
The Jα helix is anchored to the β structure (the core LOV2 domain) by two H-bond networks the first involving Lys533 (Jα) and the couple Glu475, Gln497 (β structure), the second involving Lys413 (Jα) and Thr535 (β structure). Moreover, Glu475 and Lys533 form an extensive hydrogen-bonding network between the core LOV2 domain and the Jα helix.
Here we plot the H-bond distances (two in the former and one in the latter).
- Bond between Gln497 and Lys533 in dark state:
- Bond between Gln475 and Lys533 in dark state:
Conclusion
Our first idea was to plot the evolution of dihedral angle, to see if it can show a change in the angle, and thus change of conformation which could elicit further process. Our plot did not show a clear movement of the helix, that's why we focused on specific residues.
GLN513 and ASN414 are involved in the stabilisation of FMN through hydrogen bonds, so it is important to study the evolution of the distance from these residues to the FMN.
Another interesting residue is the Cys450, which directly reacts with the FMN and initiates the first movement of conformational change of the protein. It allowed us to define two conformations, IN or OUT depending whether the sulfur atomin the Cys was pointing towards (respectively was not) the cofactor. Our studies gave exactly the same resutls as Halavaty and al., which was really comforting.
Finally, we studied the distance between some residues involved in the anchoring of the Jalpha helix, to see what happens to the hydrogen bonding between the LOV domain and the Jalpha helix.
These studies confirmed that our simulation is valid, and it will be further studied in the Differential Analysis part.
 "
"