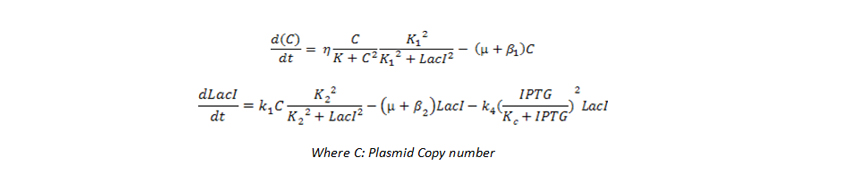Team:IIT Bombay India/Project
From 2009.igem.org
Pranayiitb (Talk | contribs) |
Pranayiitb (Talk | contribs) |
||
| Line 80: | Line 80: | ||
1. '''Steady state value of YFP v/s IPTG:''' | 1. '''Steady state value of YFP v/s IPTG:''' | ||
| - | For Strain1 and 2, increase in IPTG, does not affect the YFP. For Strain 3 and 4, increase in IPTG increases YFP expressions. | + | For Strain1 and 2, increase in IPTG, does not affect the YFP. For Strain 3 and 4, increase in IPTG increases YFP expressions. Here experimental results do not correlate with simulation results as strain 3 lies above strain 4. |
[[Image:final8.jpg]] | [[Image:final8.jpg]] | ||
''' | ''' | ||
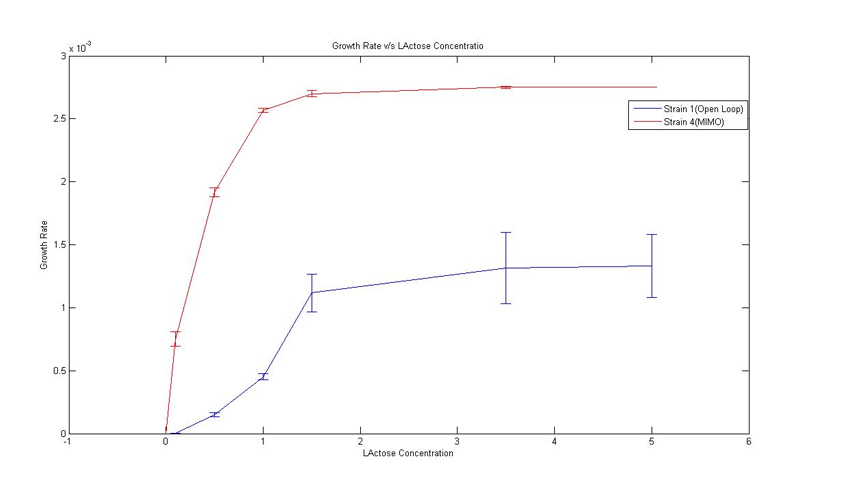
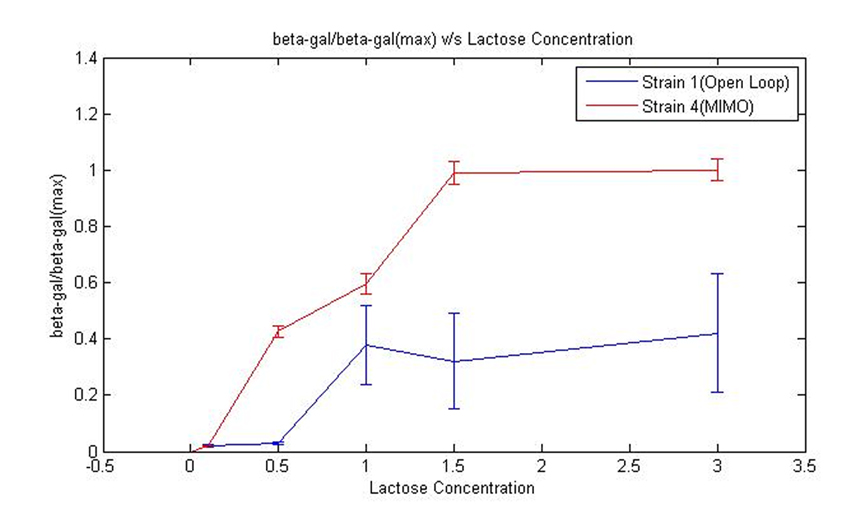
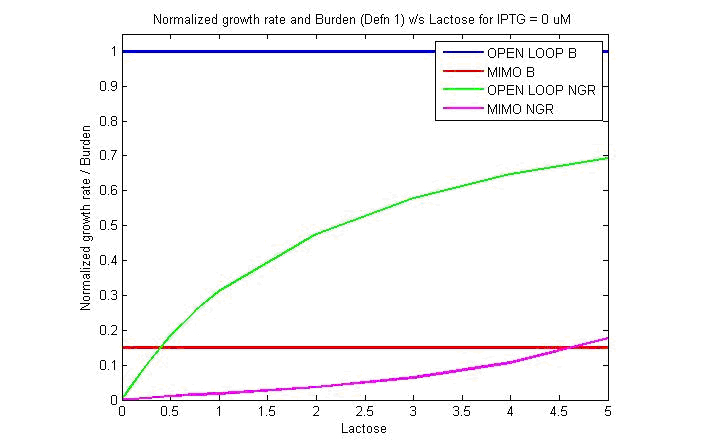
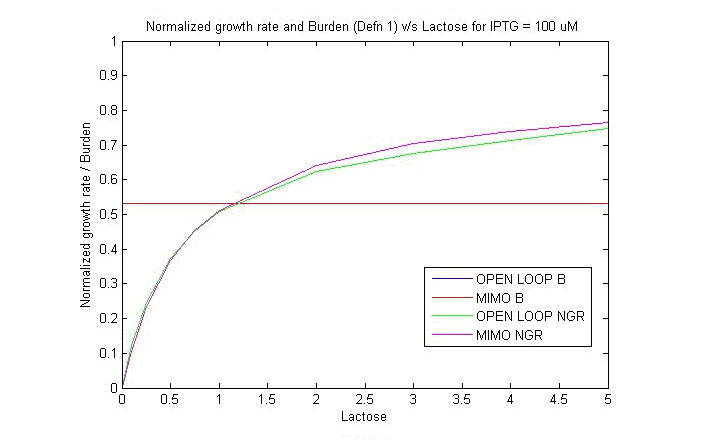
2. Growth on Lactose for Strain 1 and 4:''' | 2. Growth on Lactose for Strain 1 and 4:''' | ||
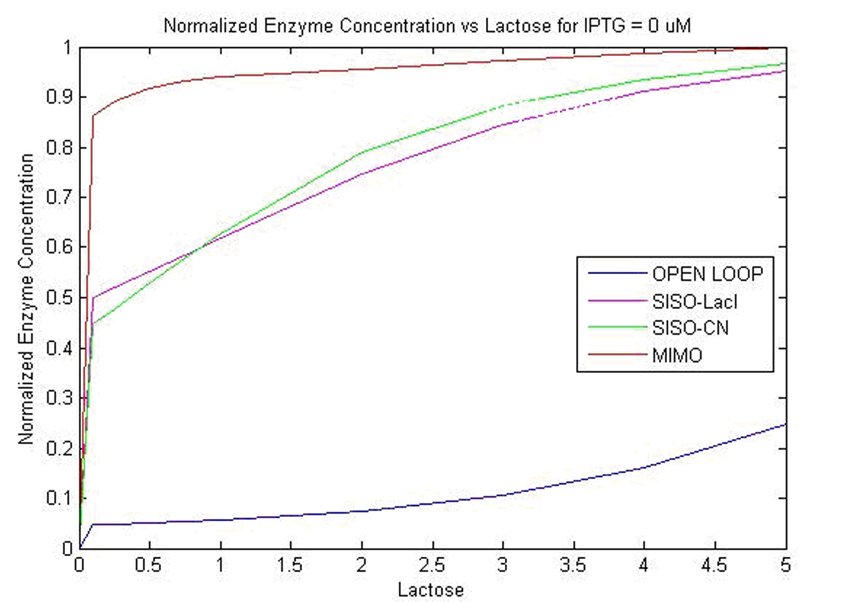
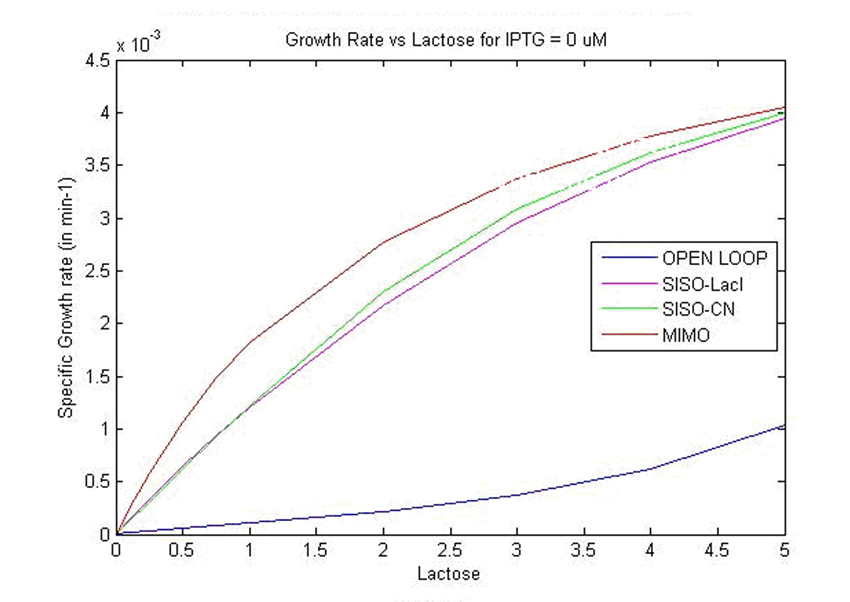
| - | We obtained specific growth rate and normalized beta-gal expression on lactose. In strain 4, we observe that | + | We obtained specific growth rate and normalized beta-gal expression on lactose. In strain 4, we observe that standard deviation is less as compared to strain 1. We also have a comparison of the results obtained from simulation. |
[[Image:final6.jpg]] | [[Image:final6.jpg]] | ||
| Line 98: | Line 98: | ||
3. '''Agar plate experiment:''' | 3. '''Agar plate experiment:''' | ||
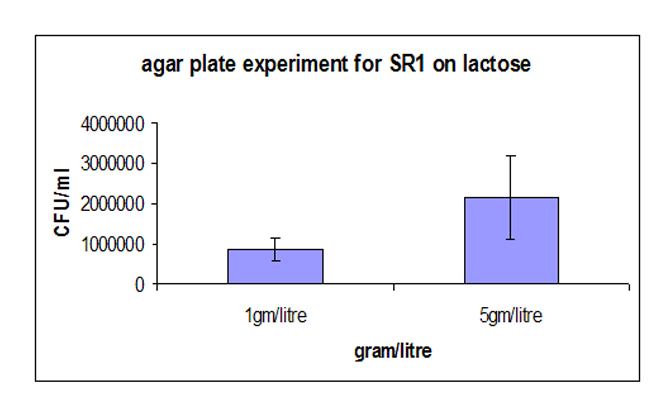
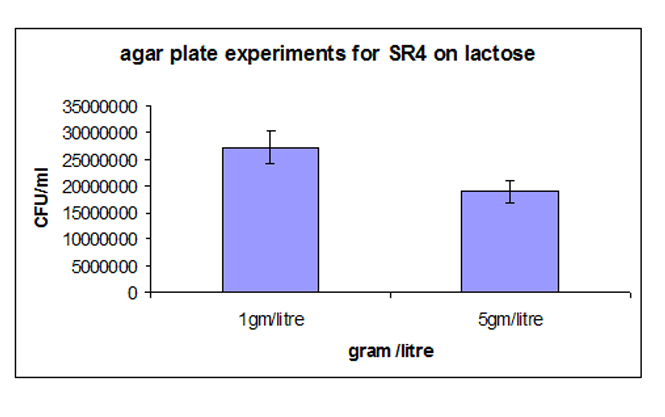
| - | Strain-4 demonstrated higher colony forming unit as compared to strain-1. The increase was about 40%. The interesting fact was that the deviation observed in Strain-4 was less compared to that of Strain-1, reiterating the fact that the noise at the protein level was translated to the phenotypic level | + | Strain-4 demonstrated higher colony forming unit as compared to strain-1. The increase was about 40%. The interesting fact was that the deviation observed in Strain-4 was less compared to that of Strain-1, reiterating the fact that the noise at the protein level was translated to the phenotypic level. |
[[Image:final4.jpg]] | [[Image:final4.jpg]] | ||
| Line 173: | Line 173: | ||
| - | Further updates and analysis will be presented [http://www.che.iitb.ac.in/online/people/faculty/core-faculty/k-v-venkatesh/igem-2009-updates|here]. | + | Further updates and analysis will be presented [[http://www.che.iitb.ac.in/online/people/faculty/core-faculty/k-v-venkatesh/igem-2009-updates|here]]. |
| - | + | ||
| | | | ||
|} | |} | ||
Revision as of 02:40, 22 October 2009
| Home | The Team | The Project | Analysis | Modeling | Notebook | Safety |
|---|
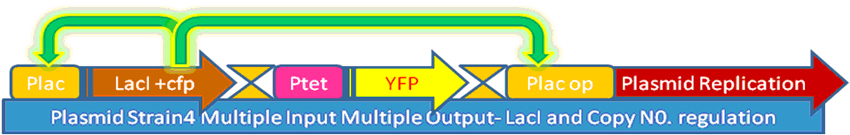
Analysis of multiple feed-backs in biological systems |
| Objective
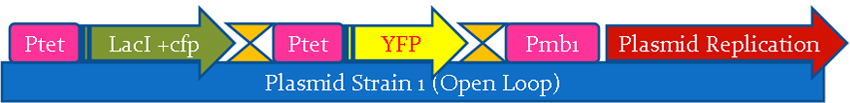
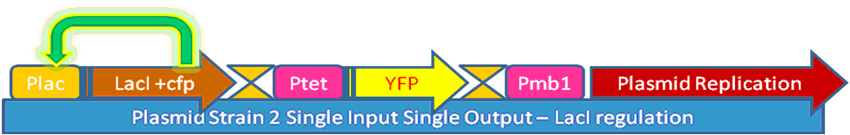
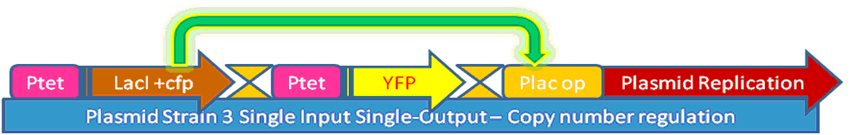
In our project, we wanted to quantify how the protein expression in a cell changes due to presence of multiple feedback loops. We developed 4 mutant strains of E. coli. Beta-galactosidase responsible for growth on lactose medium was then used to characterize the phenotypic property of specific growth rate. Experimental results were used to verify the simulated models.
Methodology
Experimental results
1. Steady state value of YFP v/s IPTG: For Strain1 and 2, increase in IPTG, does not affect the YFP. For Strain 3 and 4, increase in IPTG increases YFP expressions. Here experimental results do not correlate with simulation results as strain 3 lies above strain 4.
3. Agar plate experiment: Strain-4 demonstrated higher colony forming unit as compared to strain-1. The increase was about 40%. The interesting fact was that the deviation observed in Strain-4 was less compared to that of Strain-1, reiterating the fact that the noise at the protein level was translated to the phenotypic level. Simulations
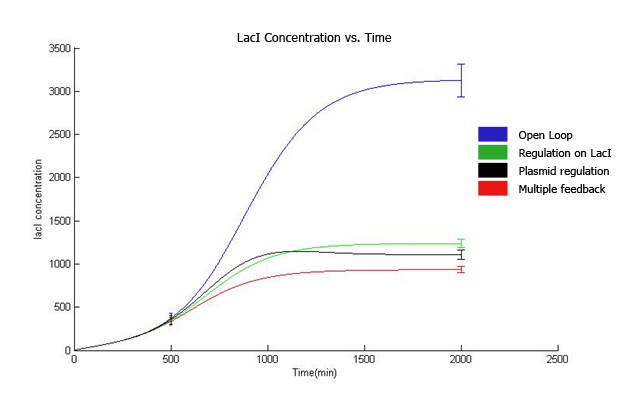
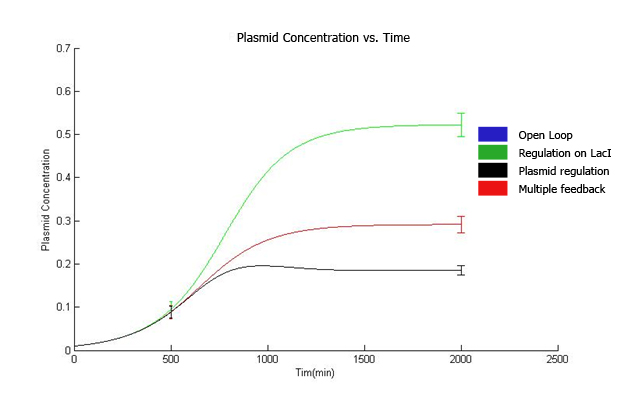
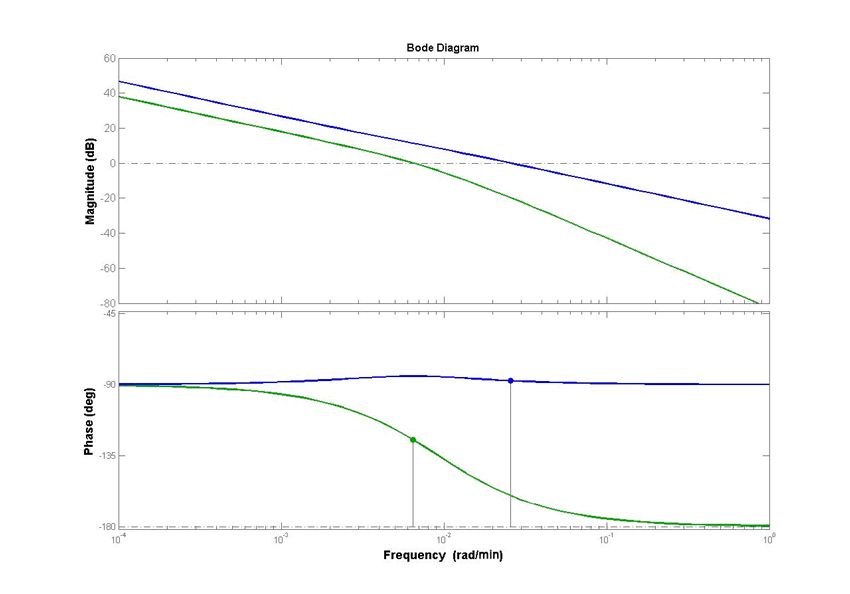
3. Control theory approach We have done frequency response analysis on the linear system using magnitude and phase Bode plots. The phase margin for strain 4 is 92.2 deg while phase margin for strain 3 is 56 deg. This indicates better ability in response to delays in production of LacI and replication of copy number. Further, we have done magnitude bode plot for the sensitivity function to determine sensitivity of the system. The bandwidth for strain 4 is 0.0255 rad/min while bandwidth for strain 3 is 0.00428 rad/min. For system with 1000 µM IPTG, the magnitude and phase bode plots are given as below: The phase margin difference is not that significant. It is 70 deg for strain 4 and 64 deg for strain 3. The magnitude bode plot for the sensitivity function shows lower bandwidth of 0.0061 rad/min for strain 4 and 0.0078 rad/min.
Future work
2. More experimentation for different values of lactose and IPTG, to get more data for beta-gal expressions and growth rate for all 4 strains 3. Detailed model: Accurately finding the kinetic constants from literature and utilizing them to accurately correlate with the experimental results; 4. Stochastic Analysis:- To develop a simplified model for growth and to introduce stochasticity in the same and characterize the inherent noise in the system. 5. Control analysis: We have done the analysis for only one kind of feedback system. We could use different feedback systems characterized by different Hill-Coefficients and try to do further analysis on the same lines as the analysis done here.
|
 "
"