Team:IPN-UNAM-Mexico/Parts
From 2009.igem.org
m (→50pxResults) |
m (→50pxResults) |
||
| (11 intermediate revisions not shown) | |||
| Line 111: | Line 111: | ||
==[[Image:Month-icon.png | 50px]]Plasmids== | ==[[Image:Month-icon.png | 50px]]Plasmids== | ||
| + | |||
| + | Check registry for more info with the name of the biobrick | ||
| + | |||
| + | |||
| + | <center> | ||
| + | {| border="1" | ||
| + | ! Status | ||
| + | ! Description | ||
| + | ! Image | ||
| + | |- | ||
| + | | SENT | ||
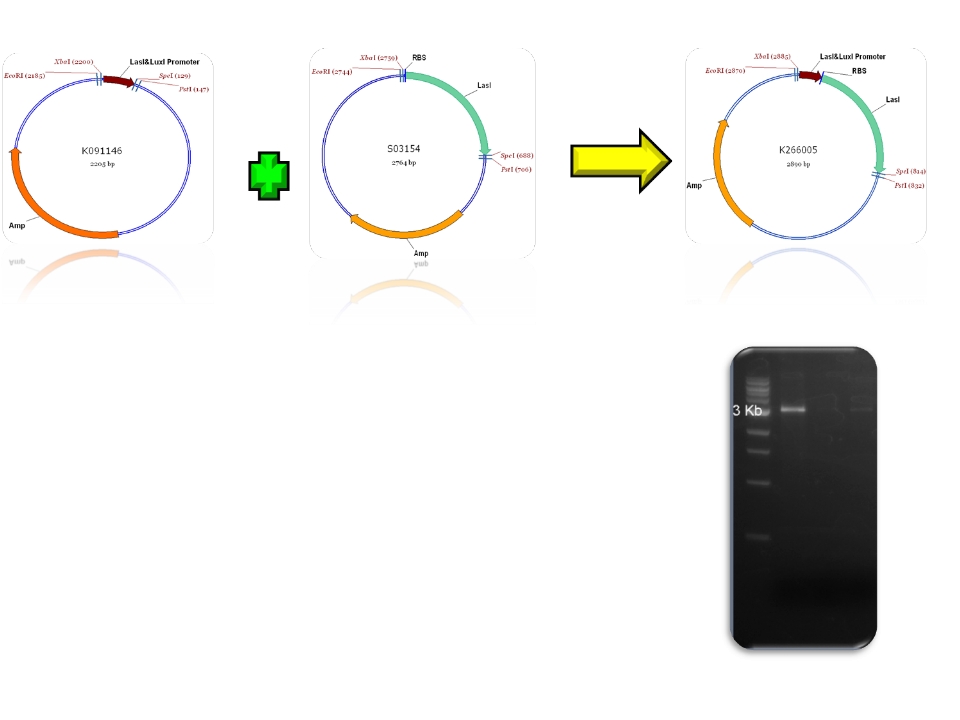
| + | | BBa_K091146 & BBa_S03154 - >BBa_K266005 | ||
| + | | [[Image:AI Biobricks.jpg|200px]]] | ||
| + | |- | ||
| + | | SENT | ||
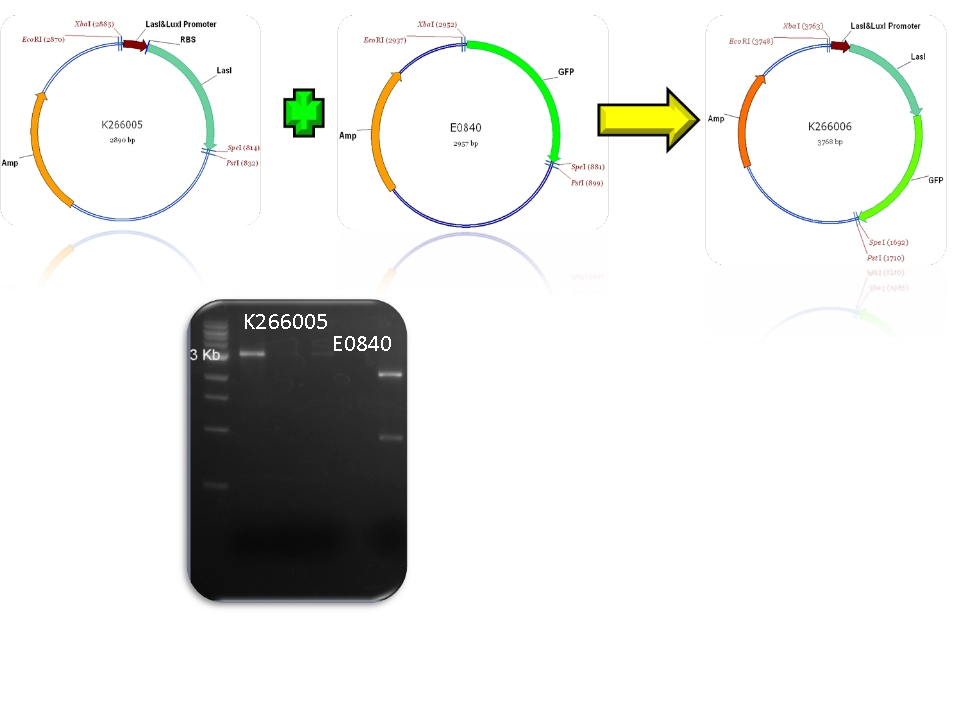
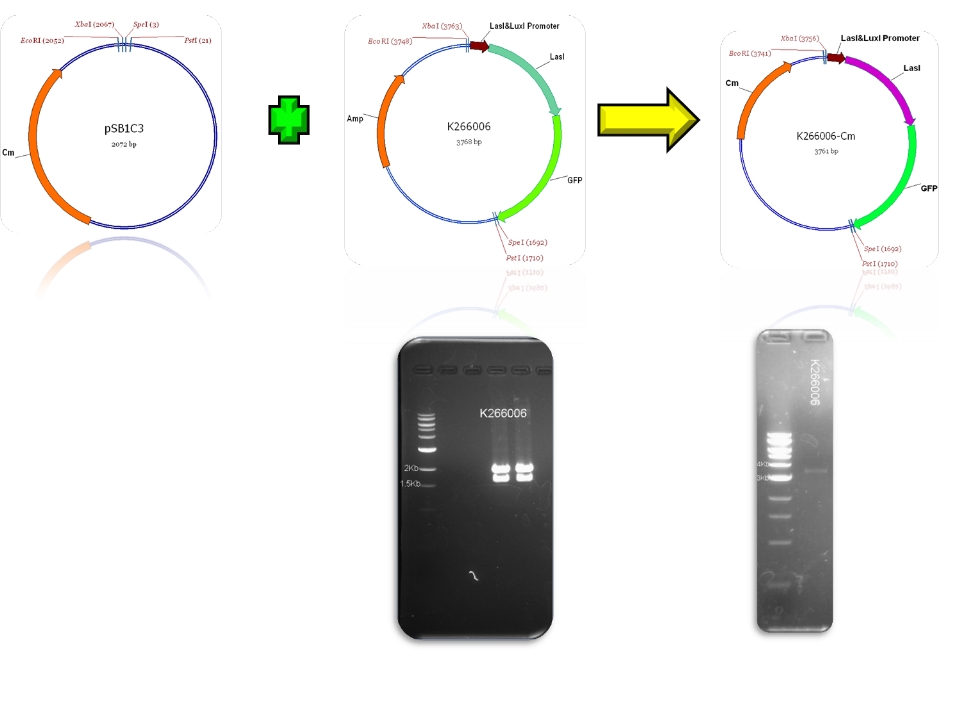
| + | | BBa_K266005 & BBa_E0840 - >BBa_K266006 | ||
| + | | [[Image:AI Biobricks2.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
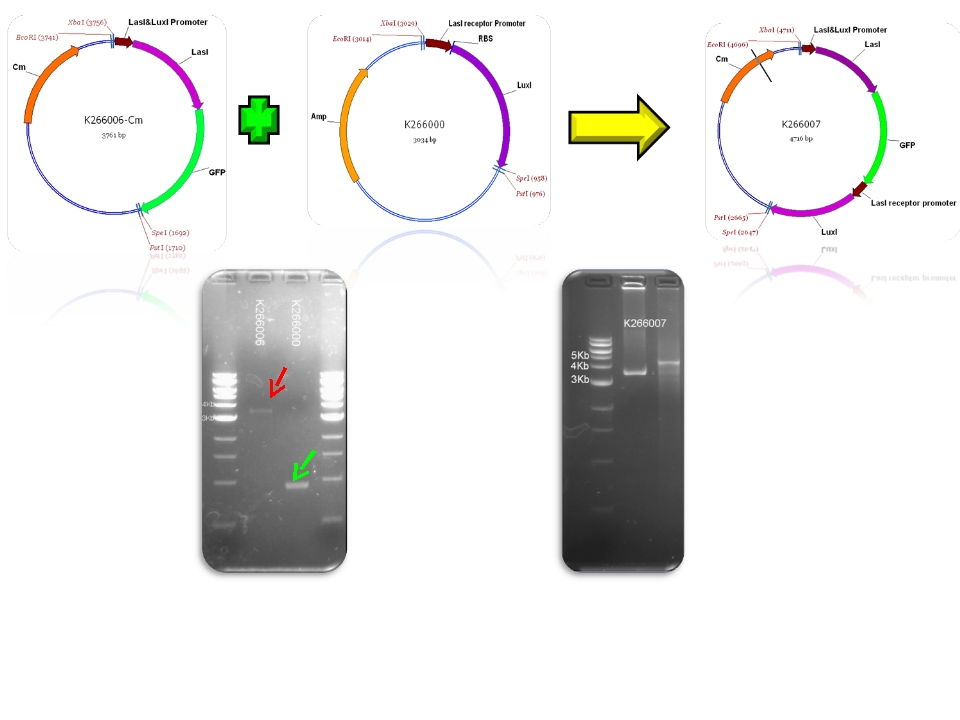
| + | | BBa_psb1c3 & BBa_K266006 - >BBa_K266006-Cm | ||
| + | | [[Image:AI Biobricks3.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
| + | | BBa_K266006-Cm & BBa_K266000 - >BBa_K266007 | ||
| + | | [[Image:AI Biobricks4.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
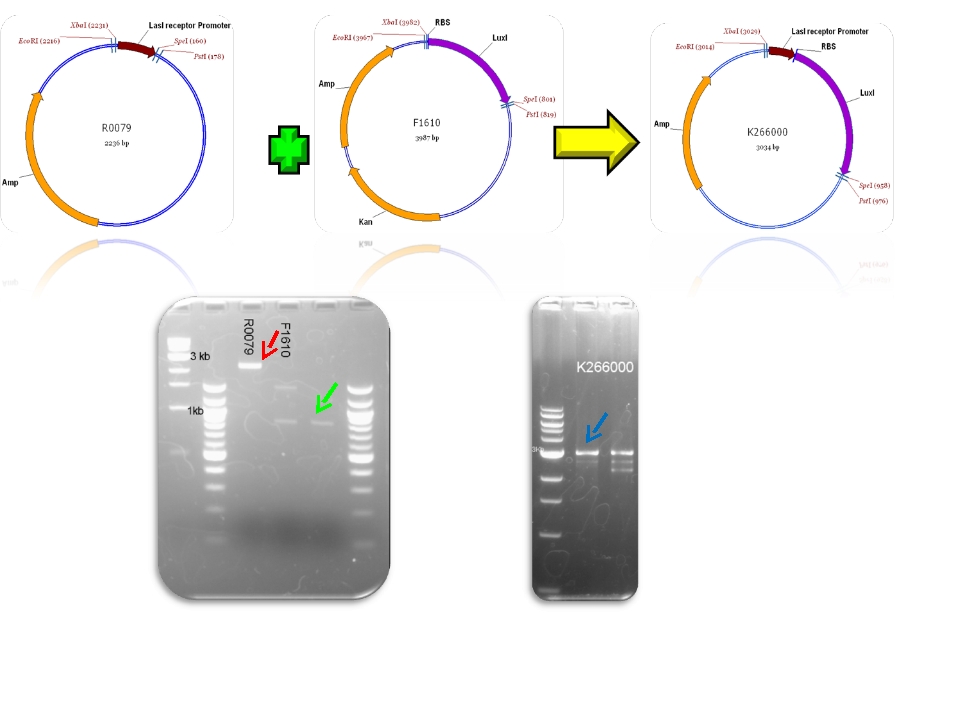
| + | | BBa_R0079 & BBa_F1610 - >BBa_K266000 | ||
| + | | [[Image:AI Biobricks5.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
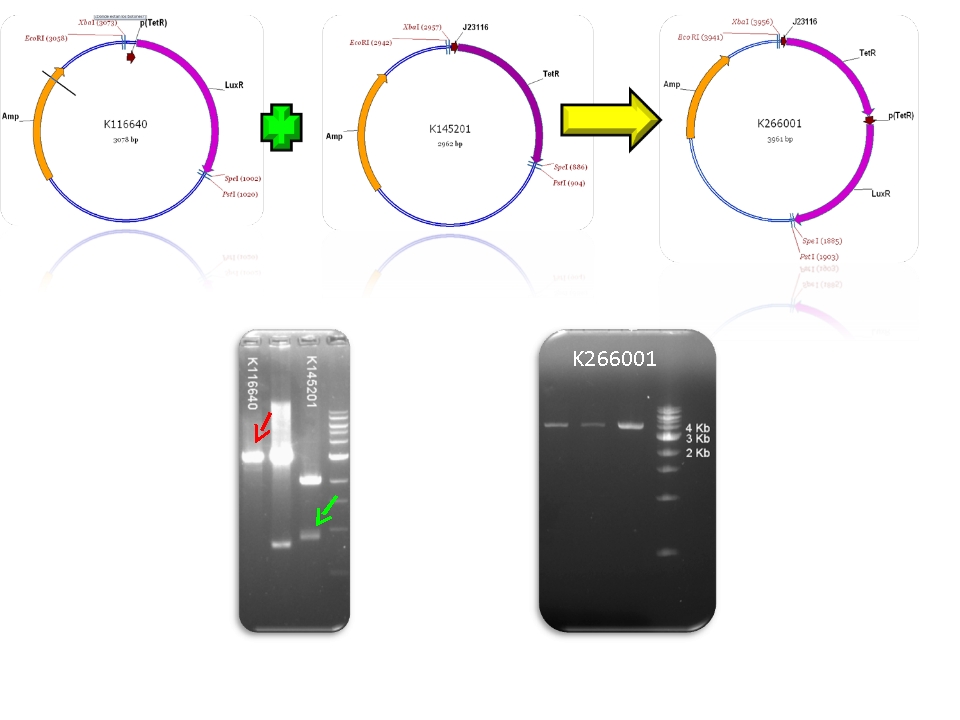
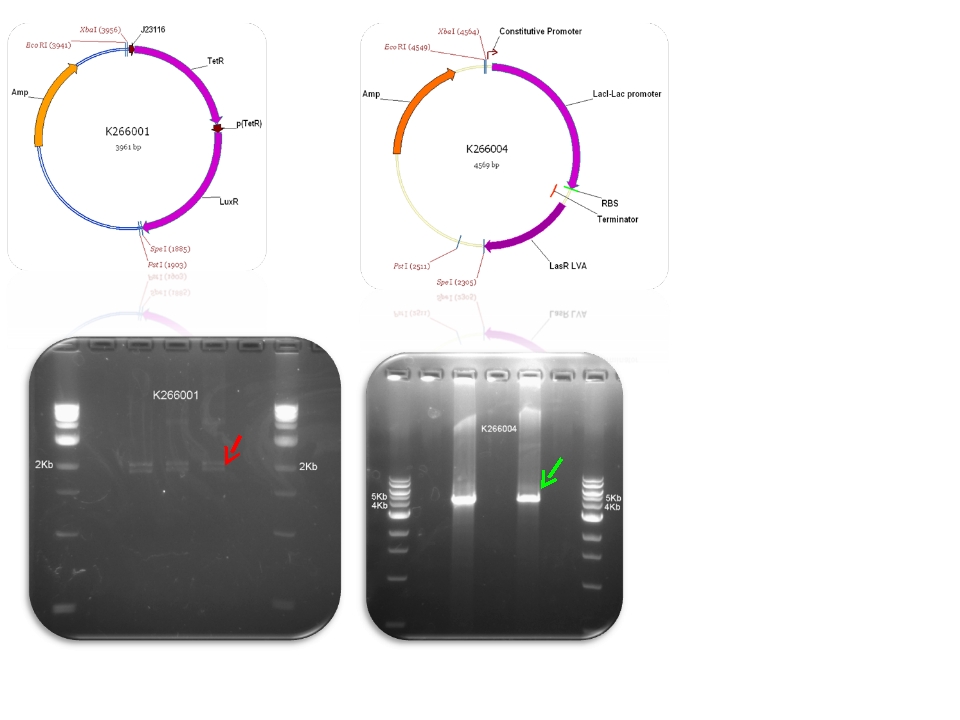
| + | | BBa_K116640 & BBa_K145201 - >BBa_K266001 | ||
| + | | [[Image:AI Biobricks6.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
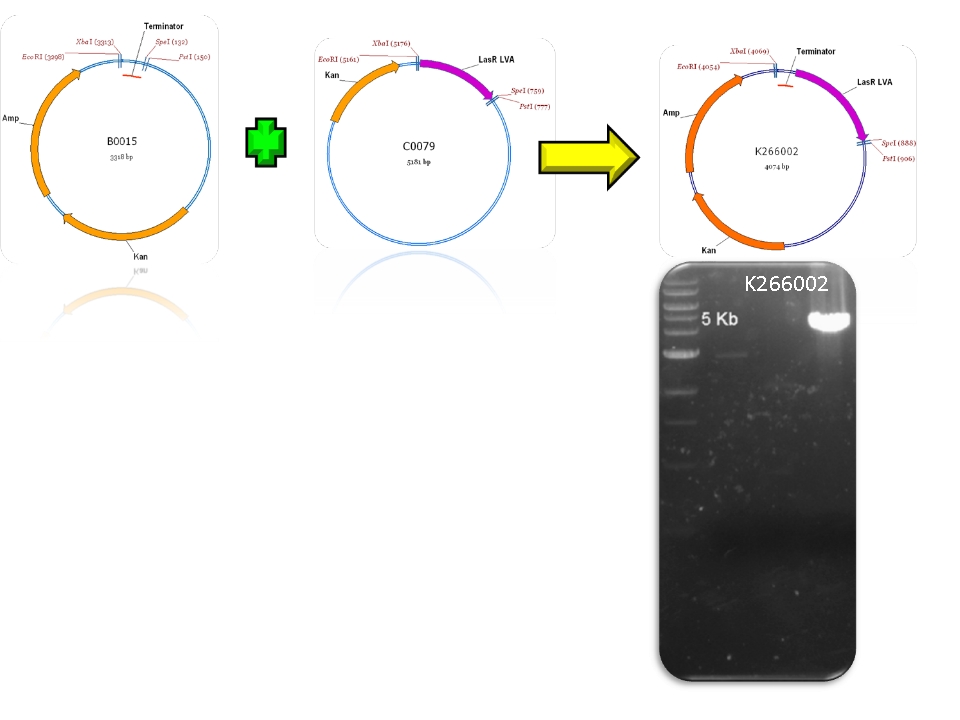
| + | | BBa_B0015 & BBa_C0079 - >BBa_K266002 | ||
| + | | [[Image:AI Biobricks7.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
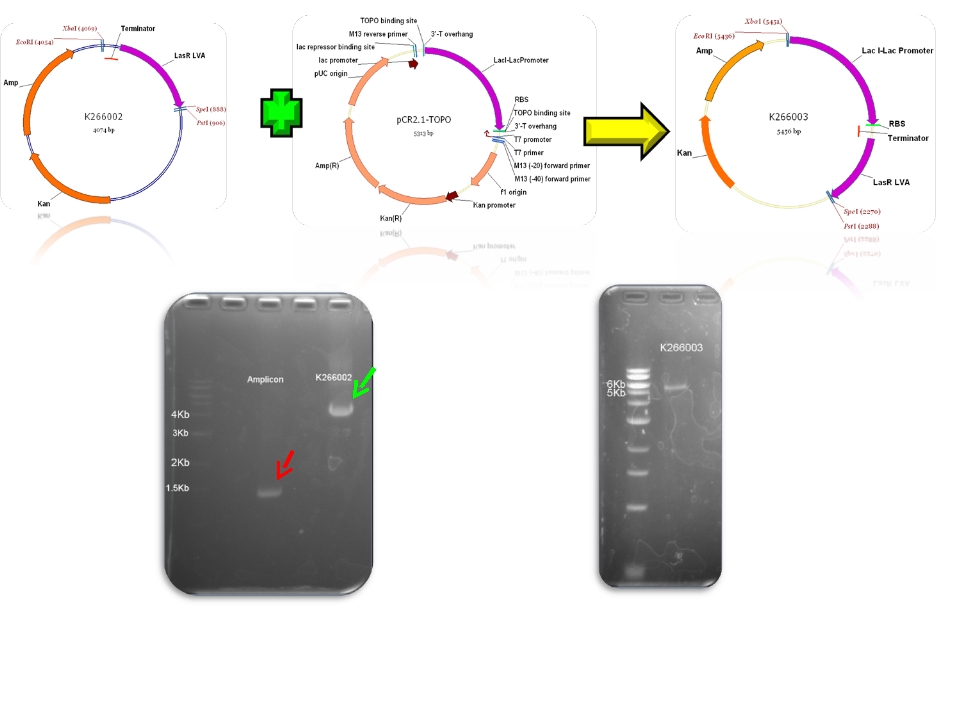
| + | | BBa_K266002 & PCR AMPLICON - >BBa_K266003 | ||
| + | | [[Image:AI Biobricks8.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
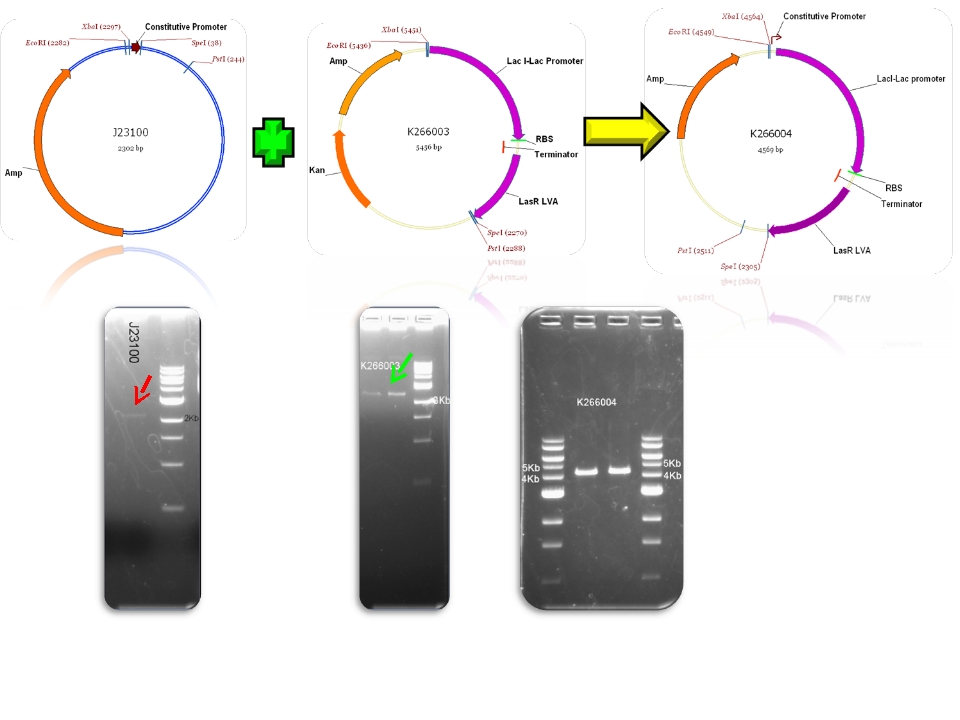
| + | | BBa_J23100 & BBa_K266003 - >BBa_K266004 | ||
| + | | [[Image:AI Biobricks9.jpg|200px]] | ||
| + | |- | ||
| + | | PENDING | ||
| + | | BBa_K266001 & BBa_K266004 - >BBa_K266011 | ||
| + | | [[Image:AI Biobricks10.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
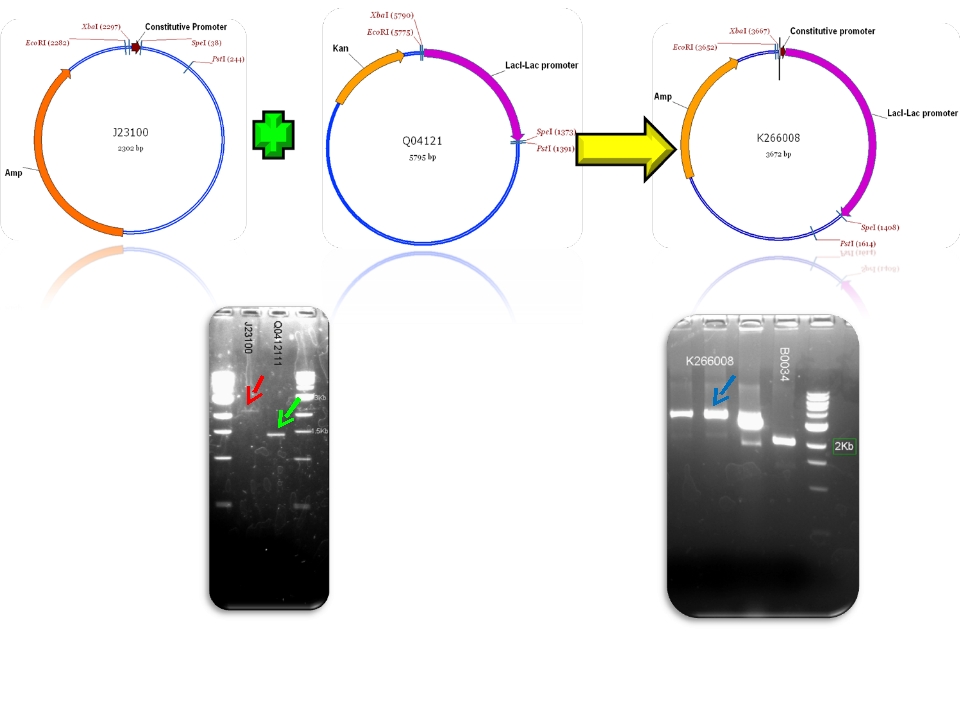
| + | | BBa_J23100 & BBa_Q04121 - >BBa_K266008 | ||
| + | | [[Image:AI Biobricks11.jpg|200px]] | ||
| + | |- | ||
| + | | SENT | ||
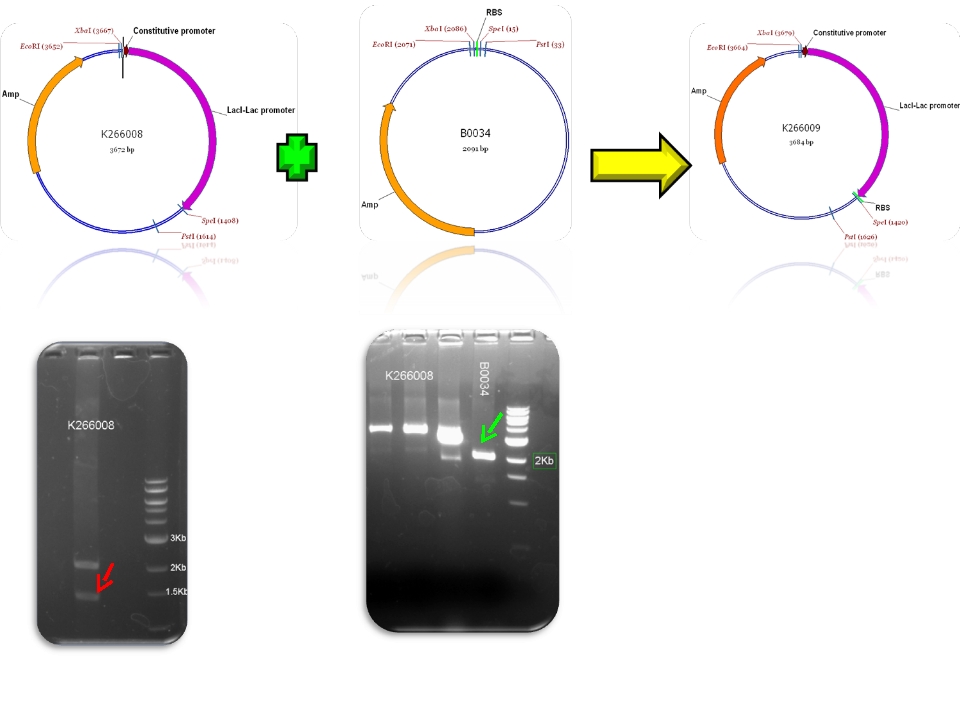
| + | | Ba_K266008 & BBa_B0034 - >BBa_K266009 | ||
| + | | [[Image:AI Biobricks12.jpg|200px]] | ||
| + | |} | ||
==[[Image:Month-icon.png | 50px]]Results== | ==[[Image:Month-icon.png | 50px]]Results== | ||
| - | [[Image:Control1.jpg | | + | As shown as follow we were able to test the viability of the Activator module coupled to its Regulatory module. |
| - | [[Image:1601.jpg | | + | |
| - | [[Image:Control2.jpg | | + | The activator and regulatory modules are composed of the following BioBricks: |
| - | [[Image:1602.jpg | | + | BBa_K266006, BBa_K266004, you can check their full description on the Modules section on the top of this page. |
| + | |||
| + | This BioBricks were into the vectors PSB1C3 y PSB1A2, with resistence to Chloramphenicol and Ampiciline respectively. The plasmids were transformed into an ''E. coli'' TOP10 strain and they were plated in agar plates containing Chloramphenicol and Amipiciline. After 24 hours at 37°C they were observed into an epifluorescence microscope Zeiss. In one plate 160mM of IPTG was previously added to the agar pate and in the other no IPTG was added. | ||
| + | |||
| + | The following pictures were taken at the optimal exitation wavelenght of the GFP (475nm) under the same exposure and amplification conditions. | ||
| + | |||
| + | e basal production of GFP that can be seen in the following pictures in basal conditions inticates that there is transcription of the BioBrick BBa_K266006. From this we can infer that that there is a basal production of LasI and thus of PAI, one of the requirements we need for our design to work properly. | ||
| + | |||
| + | As can be appreciated in the pictures with 160mM of IPTG there is an increase in the production of GFP, this indicates that the GFP was only basally expressed in the absence of IPTG, demonstrating that the Lac inversor was effectively repressing lasR, and there could be no induction in the production of GFP and LasI. | ||
| + | |||
| + | When the repression of LacI was released by adding IPTG to the media, LasR was produced and together with the basal production of PAI (by LasI) there was a positive feedback in the production of LasI, resulting in an increase of the production of both LasI and GFP (since they were under the control of the same promoter). | ||
| + | |||
| + | With this single experiment we were able to test the proper functioning of all the elements in these two modules. | ||
| + | |||
| + | {| | ||
| + | |- | ||
| + | | [[Image:Control1.jpg | 250px|center]] || Regulatory LacI module coupled to the activator module with GFP reporter module. Basal conditions. 6,4x | ||
| + | |- | ||
| + | | [[Image:1601.jpg | 250px|center]] || Regulatory LacI module coupled to the activator module with GFP reporter module. IPTG 160mM. 6,4x | ||
| + | |- | ||
| + | |[[Image:Control2.jpg | 250px|center]] || Regulatory LacI module coupled to the activator module with GFP reporter module. Basal conditions. 19x | ||
| + | |- | ||
| + | | [[Image:1602.jpg | 250px|center]] || Regulatory LacI module coupled to the activator module with GFP reporter module. IPTG 160mM. 19x | ||
| + | |- | ||
| + | |} | ||
{{Template:IPN-UNAM-Mexico-footer}} | {{Template:IPN-UNAM-Mexico-footer}} | ||
Latest revision as of 03:58, 22 October 2009
Biobricks
 Biobricks
Biobricks
We contribute to the registry with 11 new biobricks, 5 of them are favorite biobricks from the team and in this section we document them:
Main biobricks: modules and Projects
Modules
| Module and Status | Biobrick Name | Type | Image | Description |
|---|---|---|---|---|
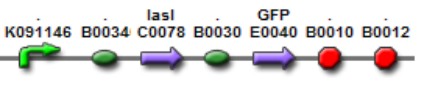
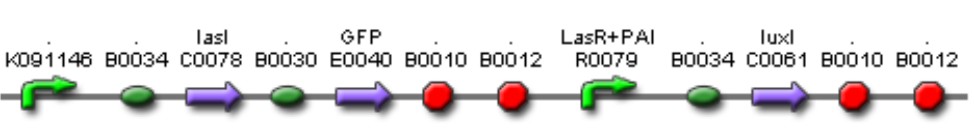
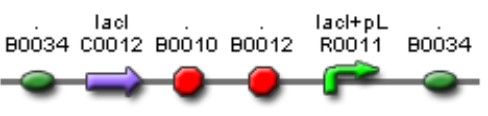
| Mod 1 Sent | [http://partsregistry.org/Part:BBa_K266006 BBa_K266006] | Las AHL | BBa_K091146 promoter (PAI+LasR inducible & AI+LuxR repressible) controls the policistronic expresion of LasI enzyme and GFP, double terminator. | |
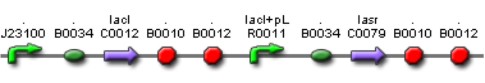
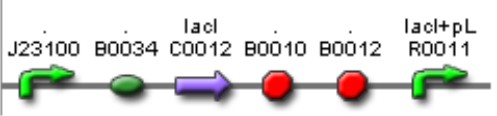
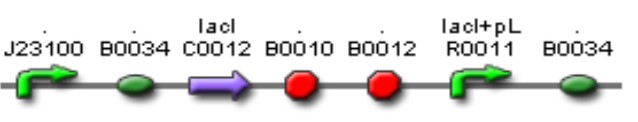
| Mod 2 Sent | [http://partsregistry.org/Part:BBa_K266004 BBa_K266004] | Lac inverter | Constitutive promoter J23100 with Lac system inverter controlling the expression of LasR. | |
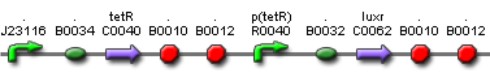
| Mod 3 Sent | [http://partsregistry.org/Part:BBa_K266001 BBa_K266001] | Tet inverter | J23100 constitutive promoter directs the expression of tetracycline repressor (TetR). TetR binds to pTet regulatory region resulting in a negative control of the production of LuxR. The whole system acts as an Inverter of Tet system controlling LuxR expression. TetR repression is inhibited by the addition of tetracycline or its analog, aTc. | |
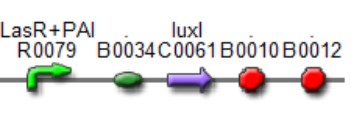
| Mod 4 Sent | [http://partsregistry.org/Part:BBa_K266000 BBa_K266000] | Lux AHL | This biobrick has a promoter inducible by PAI+LasR (BBa_R0079) I.e. positive regulation and produces LuxI enzyme (BBa_F1610). This enzyme produces 3OC6HSL (AI). |
Projects
These two biobricks are the main Activator-Inhibitor system, as showned below they are in two different plasmids, and the idea is transform Top10 cells with them in petri dishes with IPTG and ATC.
| Status | Biobrick Name | Image | Description |
|---|---|---|---|
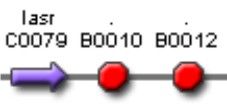
| Sent | [http://partsregistry.org/Part:BBa_K266007 BBa_K266007] | Complex Quorum sensing circuit that receives the signal of PAI+LasR and AI+LuxR to control the production of LuxI and LasI enzymes. | |
| Pending | [http://partsregistry.org/Part:BBa_K266010 BBa_K2660010] | Tet constitutive inverter controlling LasR expression and Lac constitutive inverter controlling LuxR expression. |
Auxiliary biobricks
This biobricks are though like construction intermediates necessary to build the main and project biobricks.
| Status | Biobrick Name | Image | Description |
|---|---|---|---|
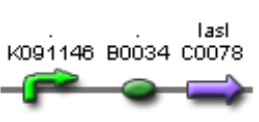
| Sent | [http://partsregistry.org/Part:BBa_K266002 BBa_K266002] | LasR coding region and a double terminator. | |
| Sent | [http://partsregistry.org/Part:BBa_K266005 BBa_K266005] | BBa_K091146 promoter (PAI+LasR inducible & AI+LuxR repressible) controls the expresion of LasI enzyme, no terminator. | |
| Sent | [http://partsregistry.org/Part:BBa_K266008 BBa_K266008] | The BBa_J23100 constitutive promoter with Lac systems inverter. | |
| Sent | [http://partsregistry.org/Part:BBa_K266009 BBa_K266009] | The BBa_J23100 constitutive promoter with Lac systems inverter and strong RBS. | |
| Sent | [http://partsregistry.org/Part:BBa_K266011 BBa_K2660011] | POPS regulated and Lac inverter system with strong RBS. |
 Plasmids
Plasmids
Check registry for more info with the name of the biobrick
 Results
Results
As shown as follow we were able to test the viability of the Activator module coupled to its Regulatory module.
The activator and regulatory modules are composed of the following BioBricks: BBa_K266006, BBa_K266004, you can check their full description on the Modules section on the top of this page.
This BioBricks were into the vectors PSB1C3 y PSB1A2, with resistence to Chloramphenicol and Ampiciline respectively. The plasmids were transformed into an E. coli TOP10 strain and they were plated in agar plates containing Chloramphenicol and Amipiciline. After 24 hours at 37°C they were observed into an epifluorescence microscope Zeiss. In one plate 160mM of IPTG was previously added to the agar pate and in the other no IPTG was added.
The following pictures were taken at the optimal exitation wavelenght of the GFP (475nm) under the same exposure and amplification conditions.
e basal production of GFP that can be seen in the following pictures in basal conditions inticates that there is transcription of the BioBrick BBa_K266006. From this we can infer that that there is a basal production of LasI and thus of PAI, one of the requirements we need for our design to work properly.
As can be appreciated in the pictures with 160mM of IPTG there is an increase in the production of GFP, this indicates that the GFP was only basally expressed in the absence of IPTG, demonstrating that the Lac inversor was effectively repressing lasR, and there could be no induction in the production of GFP and LasI.
When the repression of LacI was released by adding IPTG to the media, LasR was produced and together with the basal production of PAI (by LasI) there was a positive feedback in the production of LasI, resulting in an increase of the production of both LasI and GFP (since they were under the control of the same promoter).
With this single experiment we were able to test the proper functioning of all the elements in these two modules.
| Regulatory LacI module coupled to the activator module with GFP reporter module. Basal conditions. 6,4x | |
| Regulatory LacI module coupled to the activator module with GFP reporter module. IPTG 160mM. 6,4x | |
| Regulatory LacI module coupled to the activator module with GFP reporter module. Basal conditions. 19x | |
| Regulatory LacI module coupled to the activator module with GFP reporter module. IPTG 160mM. 19x |





 "
"