Team:IPN-UNAM-Mexico/Project
From 2009.igem.org
m (→50px The Activator-Inhibitor system on biobricks: The dynamics) |
m (→checar lode sustrato) |
||
| Line 116: | Line 116: | ||
Inspired by the Gierer and Meinhardt work we designed a synthetic genetic network that acts as an Activator-Inhibitor with limiting substrates. Our proposal is that genes of the quorum system ''las'' will have the role of the Activator, while genes of the ''lux'' quorum system are going to be the Inhibitors and ''IPTG'' and ''ATC'' will be the substrates that will delimit de pattern. We explain each particular characteristics of reaction, diffusion and substrates in the following subsections. | Inspired by the Gierer and Meinhardt work we designed a synthetic genetic network that acts as an Activator-Inhibitor with limiting substrates. Our proposal is that genes of the quorum system ''las'' will have the role of the Activator, while genes of the ''lux'' quorum system are going to be the Inhibitors and ''IPTG'' and ''ATC'' will be the substrates that will delimit de pattern. We explain each particular characteristics of reaction, diffusion and substrates in the following subsections. | ||
| - | |||
===Reaction - Diffusion=== | ===Reaction - Diffusion=== | ||
Revision as of 23:16, 21 October 2009
Turing meets synthetic biology
 Introduction
Introduction
The work of Turing
In 1952 Alan M. Turing in his classical paper called The chemical basis of morphogenesis he sugested that "...a system of chemical substances, called morphogens, reacting together and diffusing through a tissue, is adequate to account for the main phenomena of morphogenesis." This system is amazing by its simplicity. With only two morphogens it's possible to reproduce nontrivial patterns that are similar to those of zebras or leopards.
Although there is not enough evidence of the existence of these morphogens in living organisms, the likeliness of the patterns obtained by theoretical means using this model with the ones found in nature is astonishing.
Turing assumed that the morphogenes can react with each other and diffuse through cells. It is necessary that at the beginning there is a non-homogeneous distribuition of these morphogenes, which is often called chemical prepattern and can be given merely by random disturbances. An intuitive notion would tell us that the diffusion of the morphogenes starting from this chemical prepattern would led to a homogenous state of the system, but surprisingly the Turing proposal says that non-homgenous structures will arise, as a direct consequence of diffusion (the turing hyphotesis); reaching a stable state with regions with high concentrantions of one morphogen and regions with high concentration of the others. This non-homogeneous distribuition patterns of morphogens resembles those found in nature during some stages of morhpogenesis (from the gastrulation or the tentacle patterns on hydras to the jaguar spots).
Reaction-Diffusion equations
Turing formally described his proposal with a set of Partial Differential Equations where is possible to represent the chemical interactions of the morphogens and the way they move over the space. This kind of dynamics are commonly called reaction-diffusion mechanisms, and the equations that describe them are named Reaction-Diffusion equations. In section modeling we formally present them.
The Activator-Inhibitor
In 1972 A. Gierer and H. Meinhardt published his work called A theory of biological pattern formation presenting a network based in the interaction of at least two morphogenes acting as an activator and an inhibitor. The main qualitative dynamics of the morphogens is:
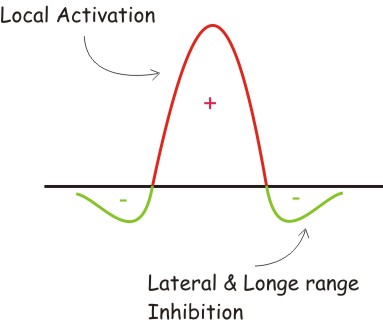
Short range activation, longer range inhibition and a conceptual distinction between effective concentrations of activator and inhibitor, on one hand, and the density of their sources on the other.
Schematically we can see this description as:
J.D. Murray presented a good example of how patterns can arise from this kind of dynamics so we cite his text literally:
Synthesizing, we can now represent the interactions between the activator and the inhibitor as follows:
The activator is autocathalyzed and it also triggers the formation of the inhibitor. Accordingly to its name, the inhibitor inhibits the production of the activator, leading to a simple but very rich dymamics (For more details on the acivator-inhibitor equations please go to modelling section). From the grass field and the fire analogy we can deduce that the diffusion coefficients of the fire should be slower than the grasshoppers one; if not the fire (activator) would spread completely in the area. This is an important fact that we mention again later.
GENERAL CONDITIONS FOR PATTERN GENERATION
Although this kind of conditions refers mainly to a mathematical analyses of the reaction-diffusion systems like the Activator-Inhibitor of J.D. Murray , we can qualitative simplify and establish according to this analyses the general conditions for pattern formation:
- The existence of at least of two morphogenes with different nature that interacts chemically between them diffuse over the space
- The coefficient rates of diffusion should be different.
- The starting distribution of morphogenes should not be completely homogeneous over the space.
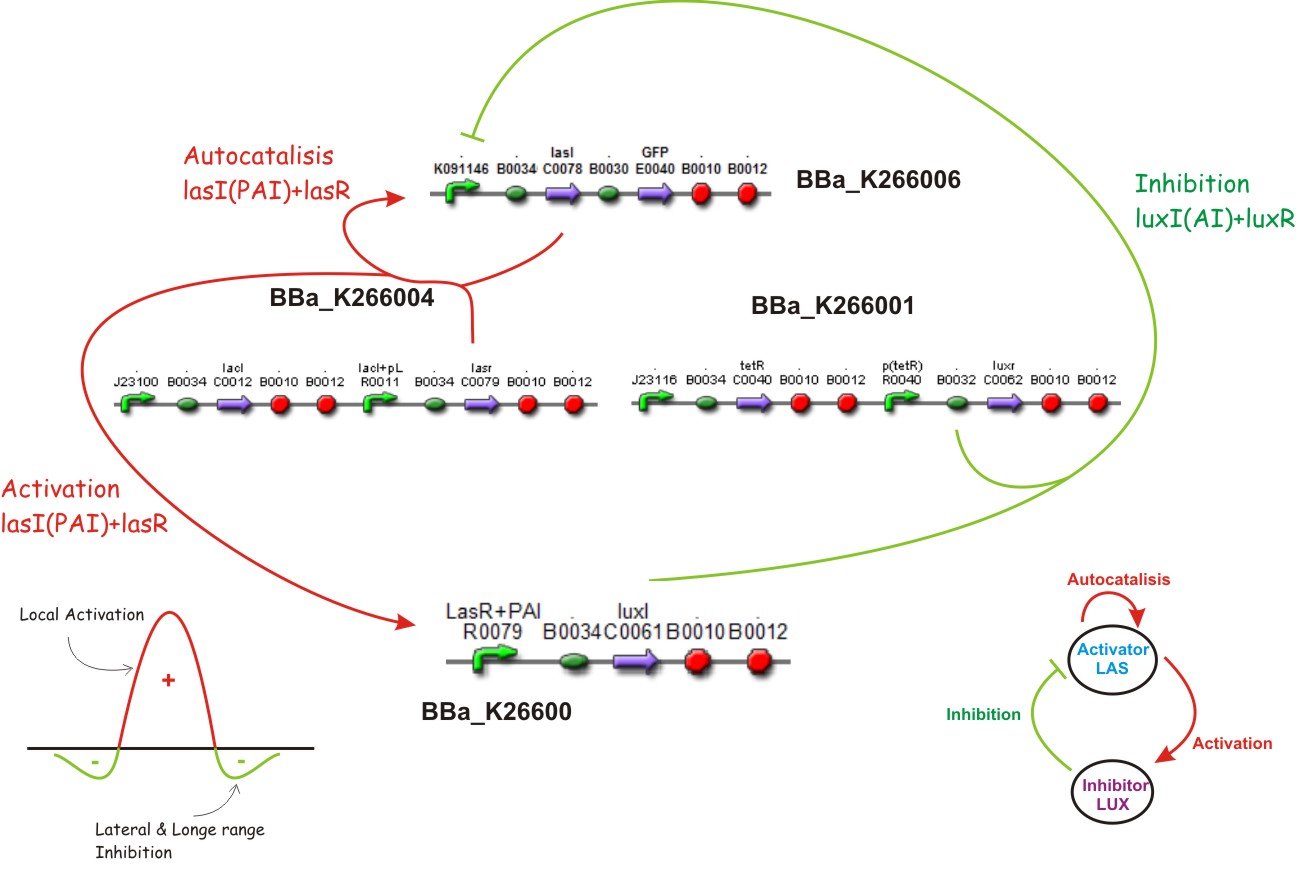
- The Gierer and Mainhardt proposal: local activation and longe range inhibition.
 TURING MEETS SYNTHETIC BIOLOGY
TURING MEETS SYNTHETIC BIOLOGY
The Synthetic Biology has became in the last years an excellent tool to designe biological systems with particular purposes. In our team we decided to used it as a way to contribute not only with technological aspects but mainly with theoretical and foundational research.
The Turing's ideas about cellular differentiation in zygote according to gradients of morphogenes that react and diffuse through the cellular tissue have not been completely confirmed due to the lack of biological evidence. The main objective of this project is to give an example of a biological system that contains reaction-diffusion mechanisms and is able to reproduce spatiotemporal Turing-type patterns or another kind of nontrivial patterns.
The biobricks found in the [http://www.partsregistry.org Registry of Biological parts] are a good starting point to construct a synthetic network of genes that due to their interactions could behave as an Activator-Inhibitor system. This genetic network inserted innto an E. coli' should respond to the concentration gradients of morphogens in the media and have the potential to accordingly differentially respond.
The main challenge was to design a synthetic network that fulfills the general conditions for generating a spatiotemporal pattern (see above). But we must act carefully, since in Murray's book literaly says :
an advise that few teams in the past years' competitions may have considered.
 Reaction-Diffusion systems implemented on Biobricks
Reaction-Diffusion systems implemented on Biobricks
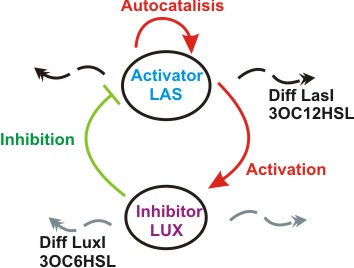
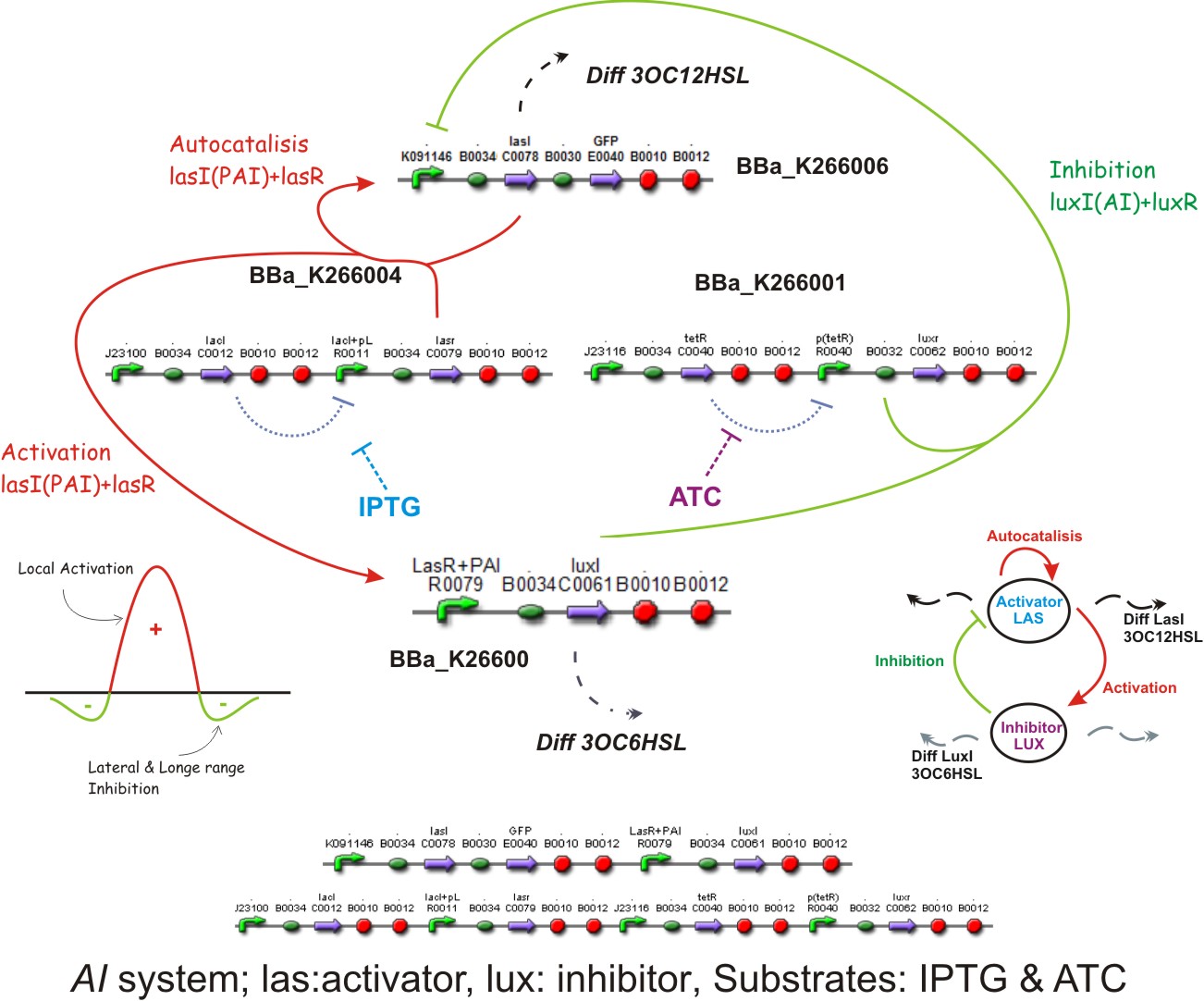
Inspired by the Gierer and Meinhardt work we designed a synthetic genetic network that acts as an Activator-Inhibitor with limiting substrates. Our proposal is that genes of the quorum system las will have the role of the Activator, while genes of the lux quorum system are going to be the Inhibitors and IPTG and ATC will be the substrates that will delimit de pattern. We explain each particular characteristics of reaction, diffusion and substrates in the following subsections.
Reaction - Diffusion
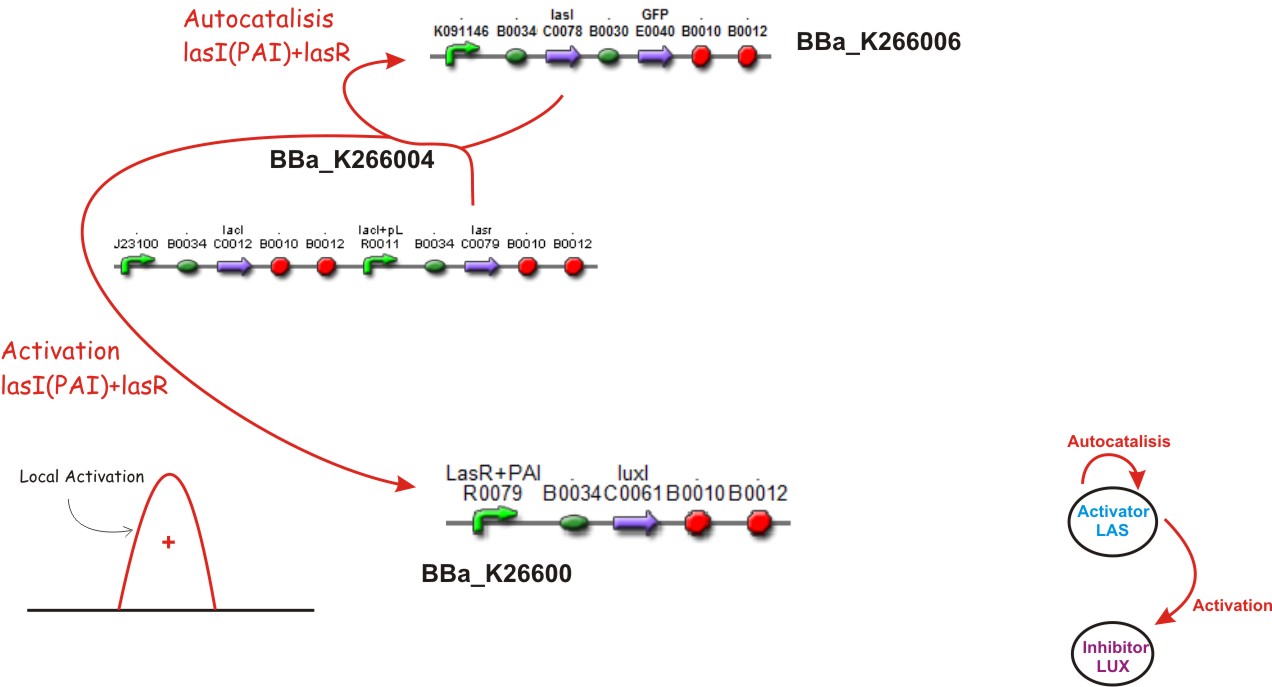
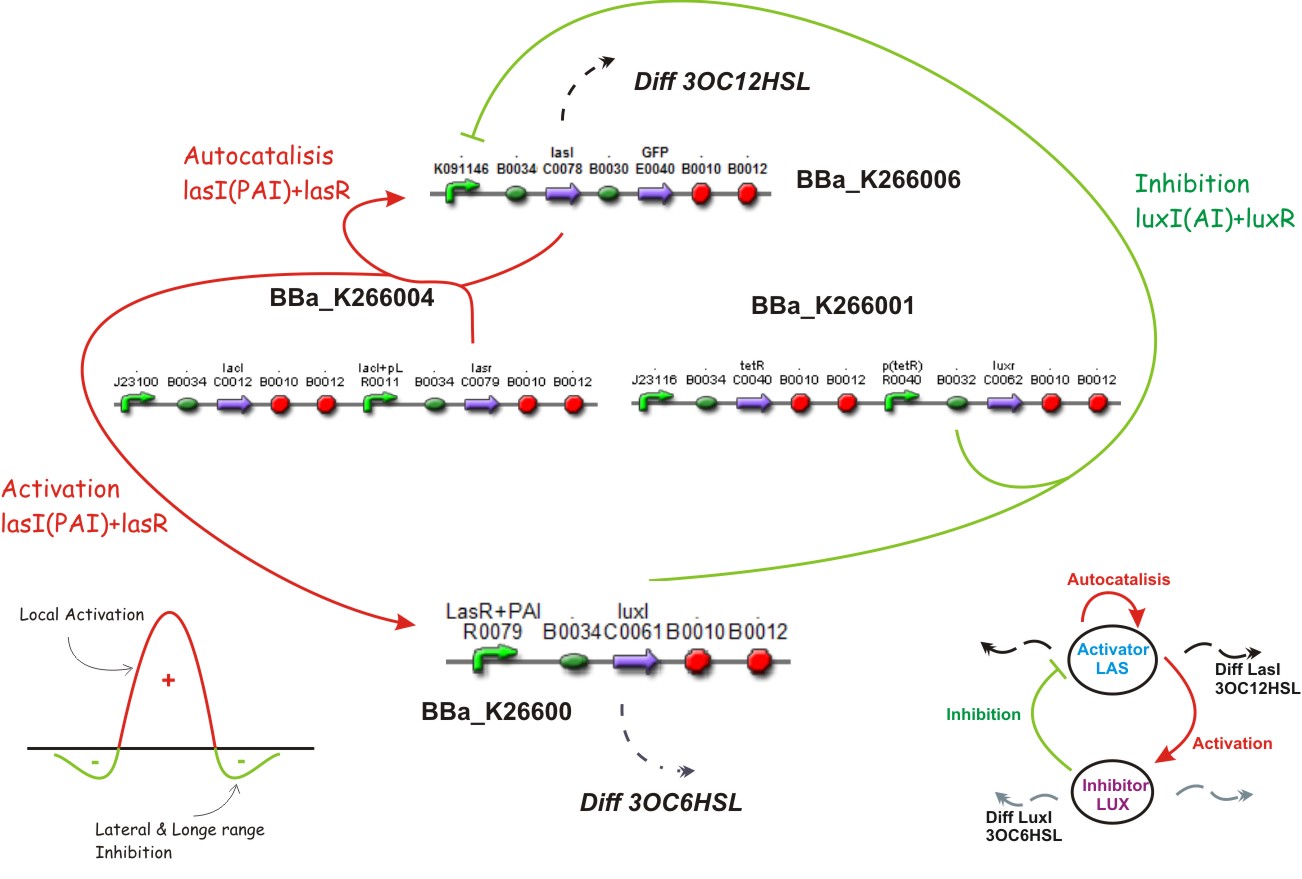
We suppose the existence of two morphogenes, represented by Lux, Las and AI, PAI lactones respectively. This compounds are very small and travel through the membrane rapidly diffusing in the media.
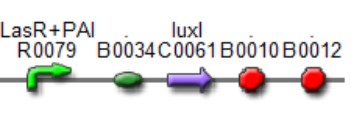
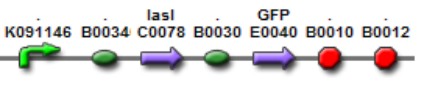
We used two promoters, one inducible by LasR+PAI ([http://www.partsregistry.org/Part:Bba_R0079 Bba_R0079]) and the other double controlled ([http://www.partsregistry.org/Part:Bba_RK091146 Bba_K091146]), repressed by LuxR+AI and induced by LasR+PAI. in our genetic network the cell will differentially respond to the different concentrations in the gradients of PAI and AI by expressing different levels of GFP.
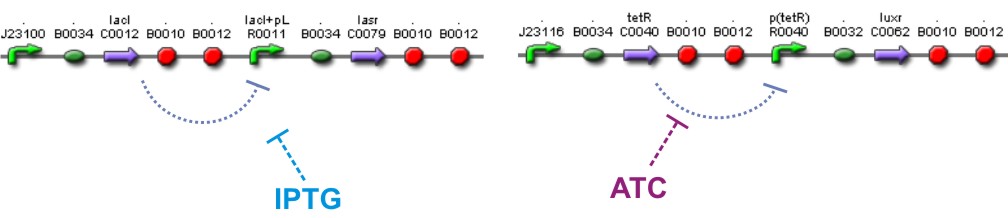
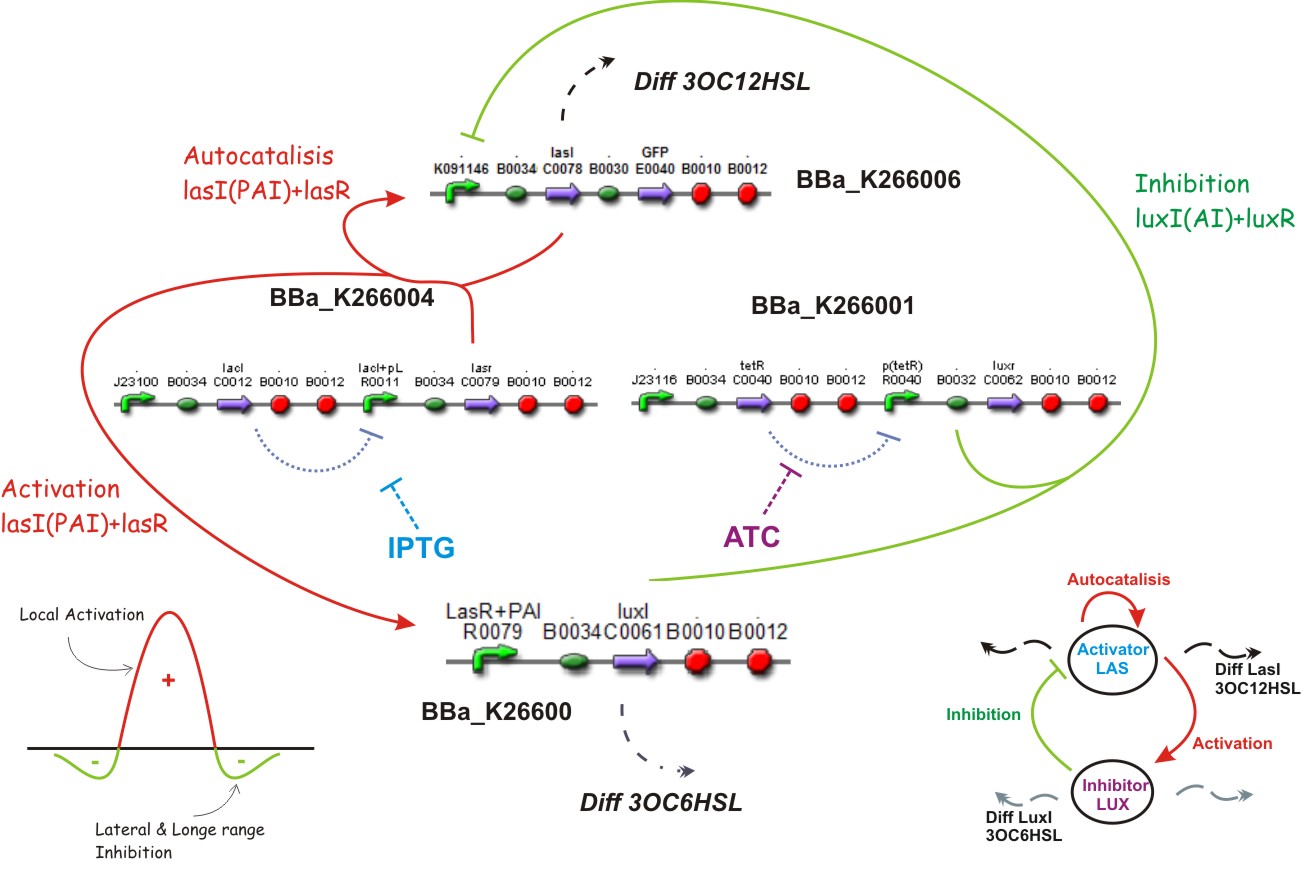
We also used a constitutive Lac inverter that allows us to control the production of LasR with IPTG and a constitutive Tet inverter that allows us to control LuxR with aTc. The controlling system of inverters provide a good way to make more sensitive the parameters adjustment.
For a complete documentation of the biobricks check parts section.
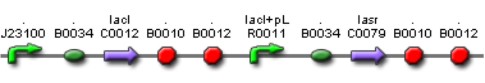
Lac inverter
The Lac Inverter is constitutive producing LacI and repressing plac promoter. Plac promoter controls de expression of LasR.
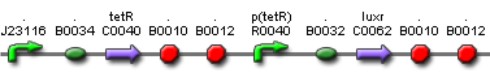
Tet inverter
The Tet Inverter is constitutive producing TetR and repressing ptet promoter. Ptet promoter controls de expression of LuxR.
Lux AHL
This biobrick is a signaling protein generator that is expected to be activated by LasR+PAI controlling the expression of LuxI enzyme.
Las AHL
This biobrick is a signaling proteing generator that is expected to be controlled in two different ways, activated by LasR+PAI and repressed by LuxR+AI. It controls the expression of LasI enzyme and GFP in a polycistronic way.
Substrates
[http://openwetware.org/wiki/IPTG IPTG] & [http://openwetware.org/wiki/ATc ATC]
Different concentrations of IPTG and ATC is expected to control too the expression of LasR and LuxR correspondly
 The Activator-Inhibitor system on biobricks: The dynamics
The Activator-Inhibitor system on biobricks: The dynamics
The questions now is, how the BioBricks system works?, in this subsection we will answer this question starting from a prototypical point of view, the activator activates the inhibitor.
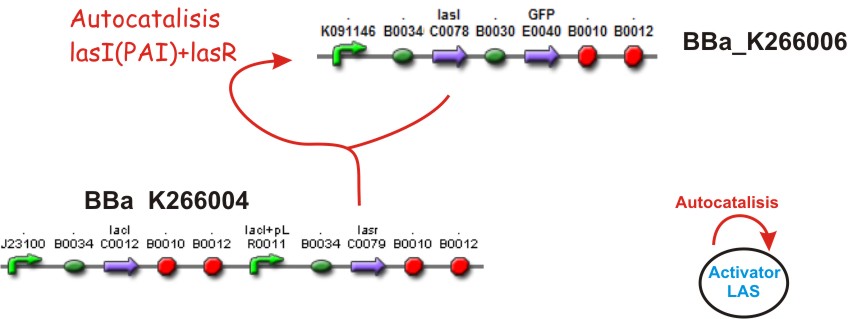
Autocatalisis
If we suppose that LacI is no produced, then the Lac promoter will produce LasR, if this happens, and the colony of E. coli is big enough to achieve a threshold of activation, a phenomena of positive feedback will start by LasR+PAI, the last one produced by LasI enzyme. This complex will induce the double promoter, the GFP will be overexpressed and much more LAS AHL will be released. A local Activation will take place.
Activation
Later LuxI enzyme will be produced by the efect of LasR+PAI on its controlling promoter, and Lux AHL will become released.
Inhibition
When there is enough Lux AHL to achieve a second threshold, and if we supose that the tet promoter is not repressed, LuxR will be produced, forming a complex with Lux AHL, LuxR + AI which in turn will repress de double promoter. The Longe range inhibition will take place.
Diffusion
Just by molecular weights, the 3OC12HSL from Las AHL and 3OC6HSL from Lux AHL, the Lux AHL will diffuse faster, (check modelling section) which covers one of the requests for generating patterns (see above).
Substrates
IPTG and ATC in the media will provide the characteristics of limiting substrates because if any of them is missing simply LuxR or LasR will not be produced because of the inverters that control them, and no interaction by quorum sensing will be possible, i.e., the reaction and diffusion part will not work and the system should arrive to an steady state.
Complete system
The two sequences from below in the picture represents the biobricks before described but in a particular order for easy work at lab only.
Resuming
The project of the IPN-UNAM-MEXICO team will provide biological evidence of a classical theoretical work of morphogenesis by means of synthetic biology. The biobricks synthetic network that we propose recover the qualitative requeriments to generate a spatiotemporal pattern because it recognize at least two differente morphogens Lux AHL and Las AHL, they diffuse with different rates, we can give a non-homogeneous starting point to the area where the morphogens will interact and diffuse with IPTG and ATC; and in areas where there are enough substrates IPTG and ATC those zones can remain activated while in a farther place the inhibitory morphogen will predomain. Hence our biobricks system is of reaction-diffusion-substrate type. For more information check de modeling, parts and results sections.





 "
"