Team:Imperial College London
From 2009.igem.org
(→Creating ‘The E.ncapsulator’: in situ manufacture and oral delivery of human biopharmaceuticals) |
|||
| Line 2: | Line 2: | ||
[[Image:II09_Encapsulator DK logo.jpg|center|The E.ncapsulator|400px]] | [[Image:II09_Encapsulator DK logo.jpg|center|The E.ncapsulator|400px]] | ||
| + | |||
| + | For iGEM 2009, the Imperial College London team present you with The E.ncapsulator. We propose a versatile manufacture and delivery platform by which therapeutics can be reliably targeted to the intestine. The E.ncapsulator represents a novel approach to drug delivery that bypasses the need for expensive purification, packaging and storage treatments. | ||
| + | |||
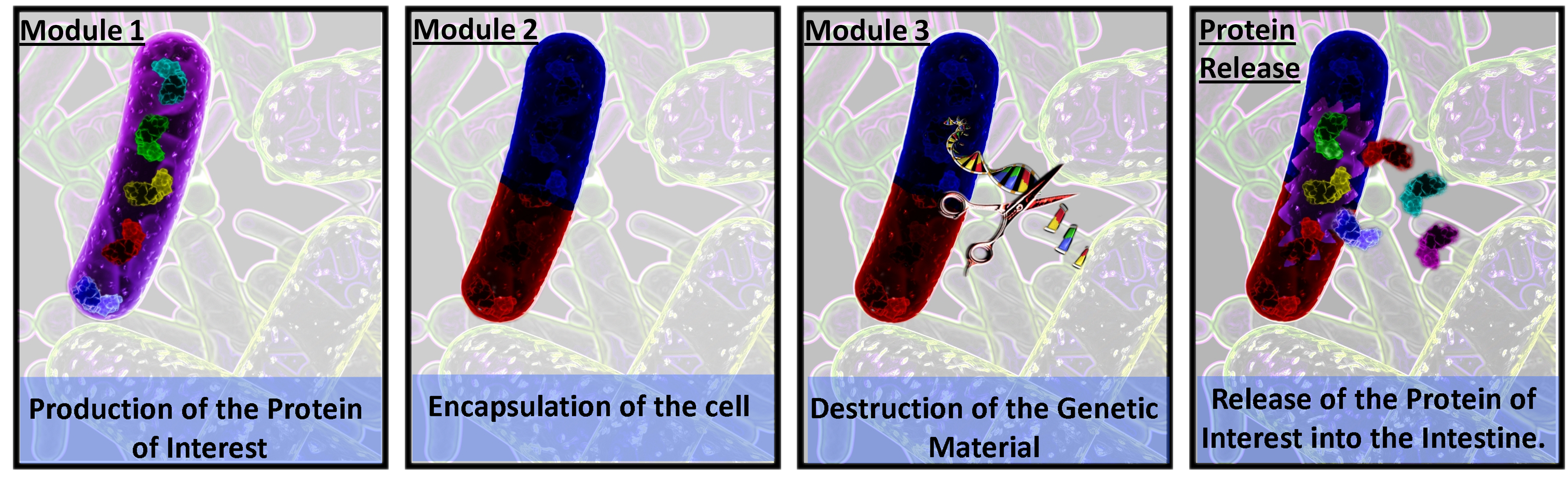
| + | Via the considered addition and modulation of genetic pathways, our E.coli chassis progresses through a series of defined stages culminating in the production of a safe, inanimate pill. This sequential process involves drug production, protective encapsulation and genome deletion. The transition through each of these stages has been industrially optimised using induction by both media and temperature. | ||
| + | |||
| + | The E.ncapsulator’s versatility is demonstrated by its ability to deliver any biologically synthesisable compound. The advantages that The E.ncapsulator offers over conventional pill manufacture makes it an attractive candidate for commercial development and additionally demonstrates the huge potential of synthetic biology in the sphere of manufacturing. | ||
Revision as of 14:37, 14 September 2009

For iGEM 2009, the Imperial College London team present you with The E.ncapsulator. We propose a versatile manufacture and delivery platform by which therapeutics can be reliably targeted to the intestine. The E.ncapsulator represents a novel approach to drug delivery that bypasses the need for expensive purification, packaging and storage treatments.
Via the considered addition and modulation of genetic pathways, our E.coli chassis progresses through a series of defined stages culminating in the production of a safe, inanimate pill. This sequential process involves drug production, protective encapsulation and genome deletion. The transition through each of these stages has been industrially optimised using induction by both media and temperature.
The E.ncapsulator’s versatility is demonstrated by its ability to deliver any biologically synthesisable compound. The advantages that The E.ncapsulator offers over conventional pill manufacture makes it an attractive candidate for commercial development and additionally demonstrates the huge potential of synthetic biology in the sphere of manufacturing.
The full project description can be found here.
 "
"