Team:NTU-Singapore/Project
From 2009.igem.org
| (28 intermediate revisions not shown) | |||
| Line 14: | Line 14: | ||
<div class="grid_3 submenu textright"> | <div class="grid_3 submenu textright"> | ||
| - | [[Team:NTU-Singapore/Project | | + | [[Team:NTU-Singapore/Project | '''p'''Laq''U''<span class="sup">e</span> '''Out''!''''']] |
| - | + | [[Team:NTU-Singapore/Project/Approach | Research Approach]] | |
| - | [[Team:NTU-Singapore/Project/ | + | |
| - | + | ||
[[Team:NTU-Singapore/Project/Prototype/Sense | Sensing Device « ]] | [[Team:NTU-Singapore/Project/Prototype/Sense | Sensing Device « ]] | ||
[[Team:NTU-Singapore/Project/Prototype/Degrade | Degradation Device « ]] | [[Team:NTU-Singapore/Project/Prototype/Degrade | Degradation Device « ]] | ||
[[Team:NTU-Singapore/Project/Prototype/Image | Imaging Device « ]] | [[Team:NTU-Singapore/Project/Prototype/Image | Imaging Device « ]] | ||
[[Team:NTU-Singapore/Project/Wetlab | Wetlab Highlights]] | [[Team:NTU-Singapore/Project/Wetlab | Wetlab Highlights]] | ||
| - | [ | + | [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2009&group=NTU-Singapore Parts Submitted] |
[[Team:NTU-Singapore/Project/References | References]] | [[Team:NTU-Singapore/Project/References | References]] | ||
</div></div> | </div></div> | ||
<div class="grid_11 suffix_1 omega"> | <div class="grid_11 suffix_1 omega"> | ||
| - | <span class="title"><span class="bold">p</span>Laq''U'' | + | <span class="title"><span class="bold">p</span>Laq''U''e <span class="bold">Out''!''</span></span><br class="clear" /> |
| Line 41: | Line 39: | ||
<b class="spiffy1"><b></b></b></b> | <b class="spiffy1"><b></b></b></b> | ||
</html></div> | </html></div> | ||
| + | |||
| + | |||
The '''NTU iGEM '09 Team''' has identified '''atherosclerosis''' as the problem we would like to tackle. | The '''NTU iGEM '09 Team''' has identified '''atherosclerosis''' as the problem we would like to tackle. | ||
| - | We propose to use a biological system that identifies and degrades atherosclerotic plaque ''in vivo''. | + | We propose to use a biological system that ''non-invasively'' identifies and degrades atherosclerotic plaque ''in vivo''. |
<br class="clear" /> | <br class="clear" /> | ||
| Line 56: | Line 56: | ||
| - | In our research and literature review, we identified the following characteristics of plaque buildup | + | '''In our research and literature review, we identified the following characteristics of plaque buildup''': |
| - | * | + | * Intuitively, we understand that decreased lumen area due to plaque leads to faster flow rate of blood. |
| + | * Damaged arterial endothelium exhibits a surface protein, '''p-Selectin''', abundantly [3]. | ||
| + | ** This p-Selectin binds selectively to the ligand, p-Selectin Glycoprotein (PSGL-1), via catch-bonds [4]. | ||
| - | * ''' | + | * Damage to arterial endothelial cells leads to decreased local levels of known vasodilator, '''Nitric Oxide''' [5]. |
| - | + | ||
| + | * The body is not able to break down '''cholesteryl esters''' in foam cells [6]. | ||
| - | |||
| - | |||
| - | + | === Present shortcomings === | |
| - | + | ||
| + | Atherosclerotic plaque buildup is a gradual process. The body accommodates for any changes, hence delaying the onset of symptoms until it's too late. Symptoms themselves vary depending on which arteries exactly the plaque buildup develop [2,8]. | ||
| - | |||
| + | As such, present medications are catered largely toward immediate relief of symptoms, and less on reversing the condition. Unfortunately, the medication is also accompanied by the need for a complete lifestyle change to prevent the problem from getting worse. More often than we would prefer, major surgeries, like bypass surgery (including open-heart surgery) & stent placement, become necessary. These surgeries are followed by long recovery periods, which may be devastating to patients. | ||
| - | |||
| - | + | === Engineering a solution === | |
| + | Our proposed biological system seeks to overcome the shortcomings of present approaches, by being less intrusive & more efficient. | ||
| - | |||
| + | [[Image:NTUplaqueout2.jpg|center]] | ||
| - | + | We propose the use of mammalian T-helper cells as a chassis. The advantage of using a T-helper cell is that expression/exhibition of PSGL-1 at the surface becomes trivial [3]. | |
| - | |||
| + | '''The chassis will contain the following devices working in synchrony.''' | ||
| - | |||
| - | |||
| - | |||
| + | [[Image:NTUsysdev.gif|center|632px]] | ||
| - | |||
| + | * '''Sensing device''' will detect changes in physiological blood [NO] and regulate the other devices. | ||
| + | ** In healthy [NO] conditions, the '''Sensing device''' represses the other '''devices'''. | ||
| + | ** In low [NO], the device de-activates and the other devices activate. | ||
| - | * ''' | + | * '''Degradation device''' will secrete an enzyme to break down the lipid core. |
| + | ** Cholesteryl esterase is released to degrade cholesteryl esters. | ||
| + | * '''Imaging device''' will identify and monitor plaque sites ''in vivo''. | ||
| + | ** Infrared fluorescence protein generates a unique infrared signal. | ||
| - | * ''' | + | * '''Dilation device''' will re-generate [NO] & feedback to shutdown. |
| - | ** | + | ** Nitric oxide synthase produces NO to dilate the vessel after a programmed delay. |
| - | + | '''We will focus on designing a full-blown prototype system as a proof-of-concept, using E.Coli as our model organism, due to equipment and time limitations.''' | |
| - | + | ||
| - | + | ||
| - | + | ||
| + | Please proceed here to understand our [[Team:NTU-Singapore/Project/Approach | Research Approach]]. | ||
Latest revision as of 00:26, 22 October 2009
pLaqUe Out!
The NTU iGEM '09 Team has identified atherosclerosis as the problem we would like to tackle.
We propose to use a biological system that non-invasively identifies and degrades atherosclerotic plaque in vivo.
Background
Atherosclerosis is one of the major diseases affecting the world. In our research, up to 7.2 million people are affected by coronary artery disease alone; a majority of these are caused by atherosclerosis [1]. In most cases, diagnosis is rare, medications are not ideal and surgeries are a commonly used last resort.
Atherosclerosis is an inflammatory disease caused by the buildup and hardening of fatty deposits on arterial walls. This accumulation eventually restricts the blood flow through the affected vessels [2]. Here's an [http://www.youtube.com/watch?v=fLonh7ZesKs informative video] describing the nature of atherosclerotic buildup, and the deadly consequences.
In our research and literature review, we identified the following characteristics of plaque buildup:
- Intuitively, we understand that decreased lumen area due to plaque leads to faster flow rate of blood.
- Damaged arterial endothelium exhibits a surface protein, p-Selectin, abundantly [3].
- This p-Selectin binds selectively to the ligand, p-Selectin Glycoprotein (PSGL-1), via catch-bonds [4].
- Damage to arterial endothelial cells leads to decreased local levels of known vasodilator, Nitric Oxide [5].
- The body is not able to break down cholesteryl esters in foam cells [6].
Present shortcomings
Atherosclerotic plaque buildup is a gradual process. The body accommodates for any changes, hence delaying the onset of symptoms until it's too late. Symptoms themselves vary depending on which arteries exactly the plaque buildup develop [2,8].
As such, present medications are catered largely toward immediate relief of symptoms, and less on reversing the condition. Unfortunately, the medication is also accompanied by the need for a complete lifestyle change to prevent the problem from getting worse. More often than we would prefer, major surgeries, like bypass surgery (including open-heart surgery) & stent placement, become necessary. These surgeries are followed by long recovery periods, which may be devastating to patients.
Engineering a solution
Our proposed biological system seeks to overcome the shortcomings of present approaches, by being less intrusive & more efficient.
We propose the use of mammalian T-helper cells as a chassis. The advantage of using a T-helper cell is that expression/exhibition of PSGL-1 at the surface becomes trivial [3].
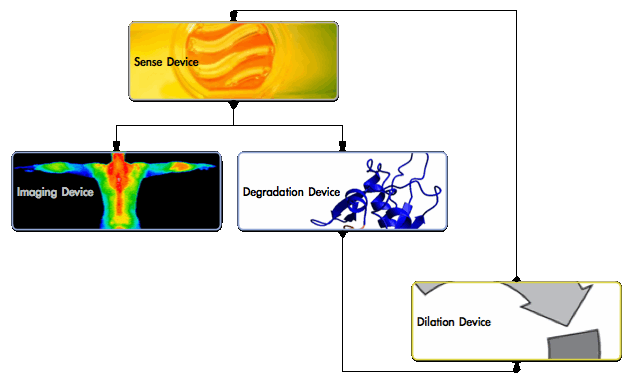
The chassis will contain the following devices working in synchrony.
- Sensing device will detect changes in physiological blood [NO] and regulate the other devices.
- In healthy [NO] conditions, the Sensing device represses the other devices.
- In low [NO], the device de-activates and the other devices activate.
- Degradation device will secrete an enzyme to break down the lipid core.
- Cholesteryl esterase is released to degrade cholesteryl esters.
- Imaging device will identify and monitor plaque sites in vivo.
- Infrared fluorescence protein generates a unique infrared signal.
- Dilation device will re-generate [NO] & feedback to shutdown.
- Nitric oxide synthase produces NO to dilate the vessel after a programmed delay.
We will focus on designing a full-blown prototype system as a proof-of-concept, using E.Coli as our model organism, due to equipment and time limitations.
Please proceed here to understand our Research Approach.
References/Literature
- WHO Medical Centre (2009). "Cardiovascular diseases (CVDs)." from http://www.who.int/mediacentre/factsheets/fs317/en/index.html.
- MayoClinic.com. (2008). "Definition of Arterioscleriosis." from http://www.mayoclinic.com/health/arteriosclerosis-atherosclerosis/DS00525.
- Eric Borges, W. T., Martin Steegmaier,* Thomas Moll,* Rupert Hallmann,§ Alf Hamann, and Dietmar Vestweber* (1997). "P-Selectin Glycoprotein Ligand-1 (PSGL-1) on T Helper 1 but Not on T Helper 2 Cells Binds to P-Selectin and Supports Migration into Inflamed Skin" Journal of Experimental Medicine 185.
- W. Thomas, “Catch Bonds in Adhesion” Annu. Rev. Biomed. Eng., vol 10, 39-57, 2008.
- Ferenc I. Tarr MD, P., Mária Sasvári MDb, Márton Tarr MD and Rozália Rácz MD (2005). "Evidence of Nitric Oxide Produced by the Internal Mammary Artery Graft in Venous Drainage of the Recipient Coronary Artery." The Annals of Thoracic Surgery 80(5): 1728-1731.
- G. K. Hansson, Anna-Karin L. Robertson, C. Soderberg-Naucler, “Inflammation and Atherosclerosis” Annu. Rev. Pathol. Mech. Dis., vol 1, 297-329, 2006.
- Moniek N. Pieters, Sebastiaan Esbach, Donald Schouten, Adriaan Brouwer, Dick L. Knook, Theo J. C. Van Berkel, "Cholesteryl esters from oxidized low-density lipoproteins are in vivo rapidly hydrolyzed in rat kupffer cells and transported to liver parenchymal cells and bile", Hepatology Vol 19, Issue 6 , Pages 1459 - 1467
- NIH. "How is Atherosclerosis Diagnosed?", from http://www.nhlbi.nih.gov/health/dci/Diseases/Atherosclerosis/Atherosclerosis_Diagnosis.html.
- E. Galkina, K. Ley, “Immune and Inflammatory Mechanisms of Atherosclerosis” Annu. Rev. Immunol., vol 27, 165-97, 2009.
- Anderson, T. J. M. (2003). "Nitric Oxide, Atherosclerosis and clinical relevance of endothelial dysfunction." Heart Failure Reviews 8: 71 - 86.
See the complete list of references here.
 "
"