Team:Newcastle/Labwork/28 August 2009
From 2009.igem.org
Formal Lab Session - 28th August 2009
Overview
- Stochastic Switch Team - ran the pSB1AT3 digest through agarose gel, excised fragment and purified. Additionally PCR reactions to amplify ara and sspb were carried out
- Metal Sensor Team - purified the excised pSB1A2 plasmid(containing BBa_J33206) and also carried out another PCR attempt on B. subtilis genomic DNA
- Promoter Library Sub-Project - ran 10ul of pGFP-rrnB treated with EcoRI and 10ul of pGFP-rrnB + NheI on some agarose gel for analysis. Additionally, 5ul of the 8Kb pGFP-rrnB digest fragment was ran on gel.
Stochastic Switch Team
Summary
Today the pSB1AT3 DNA fragment cut from the gel yesterday was purified using the gel fragment cleaning kit, and as well as this a PCR was carried out with both the ara primers on Bacillus genomic DNA, and the sspb primers on E.coli genomic DNA prepared yesterday. We expected to see a ~170bp band for the ara regulatory sequence, and a ~570bp band for the sspb coding sequence.
We also continued to extract the plasmid backbone, pSB1AT3, cut with EcoRI and SpeI sites from the gel.
PCR
PCR was carried out with both the ara primers on Bacillus genomic DNA, and the sspb primers on E.coli genomic DNA prepared yesterday. We expected to see a ~170bp band for the ara regulatory sequence, and a ~570bp band for the sspb coding sequence. The Metal team were also doing PCR work today so we made our solutions as a whole to save time and resources. Overall there were 10 reactions.
We did our two PCRs as well as negative controls that contained everything other than the genomic DNA.
- Firstly the primers had to be rehydrated and put into Phil's primer database. The primers are rehydrated in PCR water. the volume of this is 5X the 'Amount Of Oligo' (nM) which can be found on the IDT datasheet for every oligo sent to us.
- To put them into the primer database, the name must be entered, as well as the sequence, number of bp and company used. (Ask Chris to log onto the database.) The primer number can be written on the blue cap.
- After rehydration 10ul of primer can be aliquoted into separate eppendorfs from the original tube sent. This means that the original tube probably won't have to be reopened again, and can be put into storage in the primer box in the -20 freezer. The 10ul aliquots can then be diluted again to 1:10, by adding 90ul PCR water. After use the primer aliquots can be put into a separate box in the -20 also (iGEM Diluted primers 1:10).
- As there were 10 reactions overall we made up 180ul of buffer mix (sufficient for 10 reactions +2 as the protocol suggests)
Added:
- 60ul dNTPs
- 60ul buffer
- 60ul DMSO
These can be found in the 'stock solutions' box in the -20 freezer.
- We then diluted the taq Polymerase sufficient for our 10 reactions at 1 Unit per reaction. The taq stock is 5U/ul, therefore we used 3ul (enough for 15 reactions)of stock solution and 12ul PCR water.
- We entered all of our volumes of our solutions on a PCR Experiment sheet. The volume of water can be calculated by making the final volume up to 50ul.
- We labelled 4 PCR eppendorfs and added this appropriate amount of PCR water.
- Next 15ul of buffer mix was added.
- Next the primers were added- take care and keep reading the labels!
- Next the template DNA was added to the 2 tubes that are NOT controls.
- 1ul of the taq polymerase solution was added- to avoid mistakes this can be added to the master buffer solution instead.
The final solutions can be put into the PCR machine and the appropriate program set.
The PCR products can then be run on a gel which we did in the afternoon.
DNA Extraction
We took the eppendorf tube containing 184mg of gel from the fridge and solved the gel in 552ul(184*3) of gel solubilization solution. We left the tube containing the mix in 55C hot water bath for 10 minutes and vortexed every three minutes. We used two collection tubes and added 184 ul of isopropanol, 700ul of wash solution and 50ul of elute for each tube at the relevant steps. The first collection tube had 700ul of the solved gel whereas the second one had 450ul. 110ul of Elute was placed in an eppendorf tube and preheated in 65C prior to use. After adding the elute we left the tubes on the bench for a minute to incubate before the centrifuge. We got 100ul of DNA from the first tube and 80ul of from the second tube. We then combined the tubes, measured the DNA concentration and placed the tube to -20C fridge where midipreps are stored.
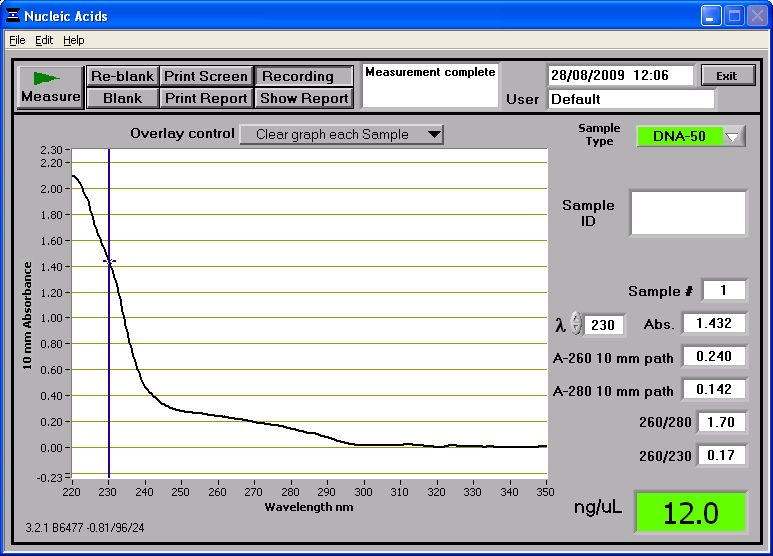
DNA Concentration
Concentration of the DNA was measured as 12ng/ul using the spectrophotometer
Metal Sensor Team
Summary
Today's lab experiment will be focussing on a second attempt to PCR the genome of Bacillus subtilis 168 cells which have taken up and inserted the pGFP-rrnB plasmid. In a couple of the previous lab sessions, the Metal Sensing team focussed on growing up some B. subtilis transformant cells (i.e. those which had taken up the plasmid pGFP-rrnB) and carrying out a PCR reaction on them using four synthesized primers to make sure that the genome carries out the insert.
Also in the last lab session, the Metal Sensing team digested 10ul of BBa_J33206 (in pSB1A2) with ' and ' and ran the fragments through agarose gel in DNA gel electrophoresis. The backbone fragment (minus the original promoter) was then excised out of the gel and stored in an Eppendorf tube. Today will see the team remove the gel from the excised band by using the Gel Extraction kit.
Practical Outline
By the time the day is over the Metal Sensing team hope to carry out the following:
- Carry out a second attempt at a PCR reaction on the genome of Bacillus subtilis
- Purify an excised band (the BBa_J33206 in the pSB1A2 plasmid without the promoter)
Procedure
PCR Reaction
Exp.No.2
We used the same protocol as used on the 21/08/2009 to carry out our PCR. The only difference is that this time we have only 6 samples, and the annealing temperature will be changed to 50 degree ( instead of the previous 56 degree). Also, we got some B.subtilis genomic DNA from Prof.Colin Hardwood's lab; last time the PCR reaction was carried out on crude cell extracts.
Sample 1 : B.subtilis 168 genomic DNA + pGFPconf2 + pGFPconf3 = control Sample 2 : B.subtilis transformed with pGFP-rrnB + pGFPconf2 + pGFPconf1 Sample 3 : B.subtilis transformed with pGFP-rrnB + pGFPconf4 + pGFPconf3 Sample 4 : B.subtilis transformed with pGFP-rrnB + pGFPconf1 + pGFPconf4 Sample 5 : B.subtilis transformed with pGFP-rrnB + pGFPconf2 + pGFPconf3 Sample 6 : PCR water + pGFPconf2 + pGFPconf3 ( no template DNA ) = control
| No. | H2O | PCR Mix | Primer Forward | Primer Reverse | Template | Pol.Used | Total Vol. |
|---|---|---|---|---|---|---|---|
| 1 | 27 | 15 | pGFPconf2 [2.5] | pGFPconf3 [2.5] | 168 DNA [2] | Moltaq [1] | 50 |
| 2 | 27 | 15 | pGFPconf2 [2.5] | pGFPconf1 [2.5] | 168withpGFPrrnB DNA [2] | Moltaq [1] | 50 |
| 3 | 27 | 15 | pGFPconf4 [2.5] | pGFPconf3 [2.5] | 168withpGFPrrnB DNA [2] | Moltaq [1] | 50 |
| 4 | 27 | 15 | pGFPconf1 [2.5] | pGFPconf4 [2.5] | 168withpGFPrrnB DNA [2] | Moltaq [1] | 50 |
| 5 | 27 | 15 | pGFPconf2 [2.5] | pGFPconf3 [2.5] | 168withpGFPrrnB DNA [2] | Moltaq [1] | 50 |
| 6 | 29 | 15 | pGFPconf2 [2.5] | pGFPconf3 [2.5] | 0 | Moltaq [1] | 50 |
PCR conditions:
1- 95C for 2min
2- 95C for 30sec
3- 50C for 30sec
4- 75C for 2min
Step 2-3-4 x 30
5- 75C for 5min
6- 4C Pause
Results
Run the PCR products on a gel at the same time as the stochastic switche's PCR products. Had No PCR products on the gel.
Excising the cut pSB1A2 band
The next task was to clean up the promoter-less BBa_J33206 plasmid by removing the surrounding agarose gel. To do this the team used Sigma-Aldrich's GenElute Gel Extraction kit along with it's protocol (found in the Technical Bulletin)
There were no changes to the protocol. There are a couple of notes to make however:
- The Spin Method was the protocol route taken.
- The weight of the gel was 434ug so for step 3a, 1302ul of Gel Solubilization Solution was added (3 times the weight of the gel)
- The amount of isopropanol added to the mixture in step 6a was therefore 434ul
Once the process was completed the DNA was transferred to the -20°C freezer for storage.
Promoter Library Sub-Project
Introduction
So far, the work involved in this sub-project has seen pGFP-rrnB plasmid DNA digested with restriction enzymes EcoRI and NheI. The resulting 8kb DNA fragment was then extracted by firstly running the digested DNA through agarose gel in electrophoresis and then by excising the heavies band formed. The fragment DNA was then extracted from this removed piece of agarose by gel extraction kit and the 50ul of resulting DNA was stored in the -20C freezer.
When running the digested pGFP-rrnB plasmid DNA though the gel to extract the 8Kb fragment, we did not see the bands expected to be seen. Therefore another restriction enzyme digestion of the pGFP-rrnB DNA with EcoRI and NheI was attempted (with different concentrations of the enzymes and DNA). In addition more pGFP-rrnB DNA was digested but with EcoRI and NheI separately. All of the resulting digests were stored in the -20C freezer.
In today's experiments we hope to run 10ul of pGFP-rrnB treated with EcoRI and 10ul of pGFP-rrnB + NheI on some agarose gel for analysis. Additionally, 5ul of the 8Kb pGFP-rrnB digest fragment should be run on the gel.
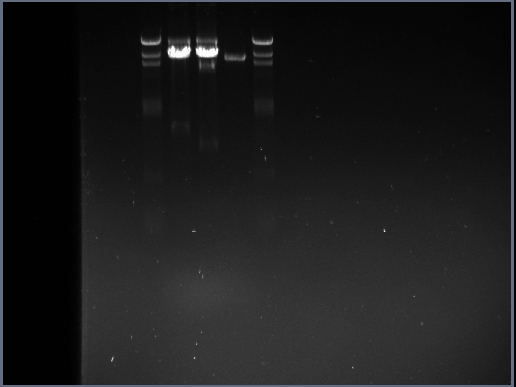
DNA gel Electrophoresis of samples
0.8% agarose gel was prepared and poured in the manner exhibited in the DNA gel electrophoresis protocol. Once this step had been carried out, the DNA samples (once dye had been added) were loaded in the wells as follows:
- Lane 1 - blank
- Lane 2 - 8ul HindIII DNA ladder
- Lane 3 - 10ul of pGFP-rrnB + EcoRI
- Lane 4 - 10ul of pGFP-rrnB + NheI
- Lane 5 - 5ul of the pGFP-rrnB 8Kb fragment
- Lane 6 - 8ul HindIII DNA ladder
The gel electrophoresis was run for 20-30 minutes under 70mv; once stopped the gel was taken to the dark room for analysis.
Resulting gel photograph
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
News
Events
- 20 – 21 June 2009 - Europe workshop (London)
- 23 – 24 June 2009 - UK iGEM meetup (Edinburgh)
- 23 October Practice Presentation (Newcastle)
- 23 October T-shirts are ready
- 27 October Practice Presentation (Sunderland)
- 27 October Poster is ready
- 30 October – 2 November 2009 - Jamboree (Boston)
Social Net
- Newcastle iGEM Twitter
- [http://www.facebook.com/home.php#/group.php?gid=131709337641 Newcastle on Facebook]
- [http://www.youtube.com/user/newcastle2009igem Newcastle Youtube Channel]
 "
"