Team:Paris/Conclusion
From 2009.igem.org
iGEM > Paris > Home > Conclusion & Results
Contents |
Results
In order to prove the feasibility of our project 5 main points needed to be adressed:
1. Addressing proteins to the sender bacteria outer membrane
2. Controlling the production of outer membrane vesicules
3. Addressing proteins to outer membrane vesicles
4. Fusing outer membrane vesicles of a sender population with the outer membrane of receiver cells
5. Transducing a signal from a protein in the outer membrane to a reporter gene in the receiver
We provided experimental evidence for at least 3 out of this 5 points:
Addressing proteins into the sender bacteria outer membrane
Our strategy was here to take advantage of the clyA outer membrane protein by fusing a fluorophore to N or C terminal of the ClyA protein.
METTRE L'IMAGE DE LA CONSTRUCTION
FAIRE LE LIEN AVEC LE DETAIL AILLEURS DANS LE WIKI
Our clyA-mRFP1 fusion was cloned under the control of a pBAD promoter (FAIRE LE LIEN AVEC LA CONSTRUCTION DANS LA REGISTRY). We can in this microscopy pictures clearly observe the membrane localization of fluorescence.
METTRE LES IMAGES
Controling vesicle production and addessing proteins into them
The strategy was here to destabilize the outer membrane by playing with the tolAR system. We either worked with tolA- strains or we tried to overexpress protein domains of tolR in order to disrupt the Tol protein complex essential for membrane stability.
METTRE LIEN VERS DETAILS ET METTRE LES IMAGES DES CONSTRUCTIONS ET LIEN VERS LA REGISTRY
As OMVs are very small (around 100nm) they are impossible to see with optical microscopy. As we did not dispose of an electron microscope we tried to see if our clyA-mRFP1 when expressed in a tolA mutant could lead to the presence of red fluorescence into vesicles. Here are images of what could be fluorescent vesicles. Nevertheless we are just above background fluorescence and more controls would be required for a real proof.
DEMANDER A ARIEL SI IL A REUSSI A FAIRE DES IMAGES DE VESICULES ET LES METTRE ICI
The next step in the development of this strategy would be the implementation of the delay system to ensure a good quality of the emitted signal as shown thanks to modeling studies :
Transducing a signal from a protein in the outer membrane to a reporter gene in the receiver
As a first proof of principle for our project, we wanted to use the FecA protein as the message. FecA is an outer membrane protein implied in ferric citrate uptake and in signal transduction leading to the expression of the fecABCED operon. The idea is here to have a sender population producing and exporting fecA into vesicles, and a receiver population deleted for fecA. In this manner, only the receiver cells having fused with vesicles would transduce a signal leading to the expression of the pFec promoter controlling a reporter gene.
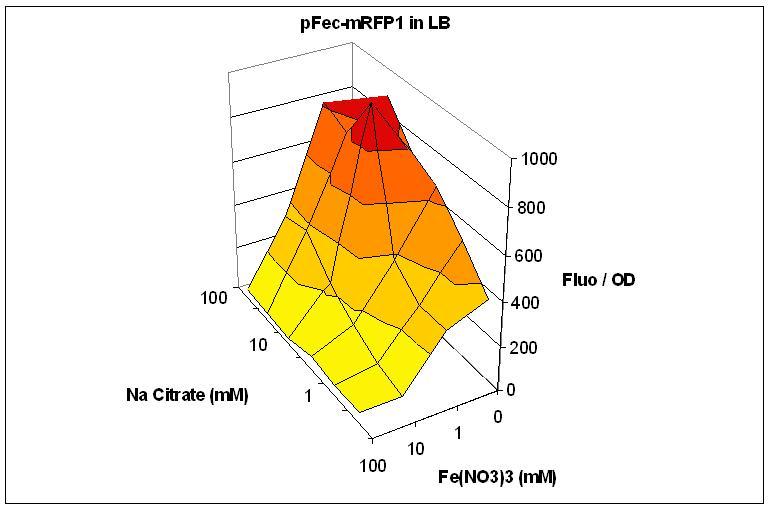
We cloned the pFec promoter into a plasmid carying the mRFP1 fluorescent protein . This allowed us to caracterize it, showing that pFec is efficiently activated when ferric citrate is present in the medium.
Overnight cultures of were diluted 1/100 in LB or M9 medium supplemented with varying amount of FeNO3 and Na Citrate. Fluorescence and OD were assessed in a Tecan plate reader. Fluorescence divided by OD is plotted. The low Fluo/OD at high FeNO3 concentration can be explained by the toxicity of FeNO3. Note that the best response to Na Citrate is obtained with 1mM FeNO3.
Conclusion
After a great summer of hard work, it's time to conclued on our project.
Concerning the laboratory experiment we learnt few things... The first one is that the bench work takes a lot of time, so even if our project is designed on paper, it's not so easy in the "practical" life.
Ethics is really important when a new discipline is created. In this direction, it was an evidence for our team to inclued the ethics as a part in our project. In this direction we were able to think about the synthetic biology and its applications. [Report Synthethics PDF]
About the collaboration, it was a great pleasure and it was reallu appreciable to work with other iGEM team for different part of our project (modelisation, ethics or i-phone tool). This collaboration allow us to contribute to the iGEM spirit.
 "
"