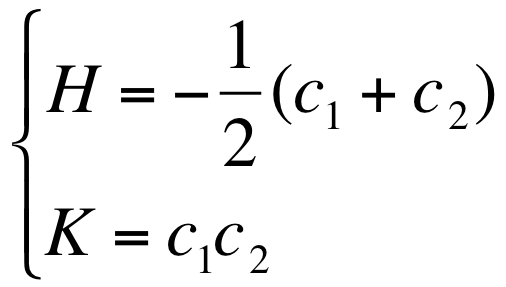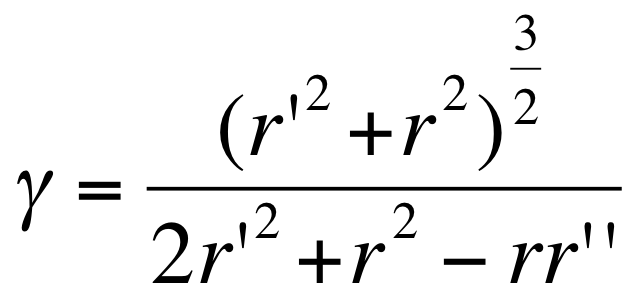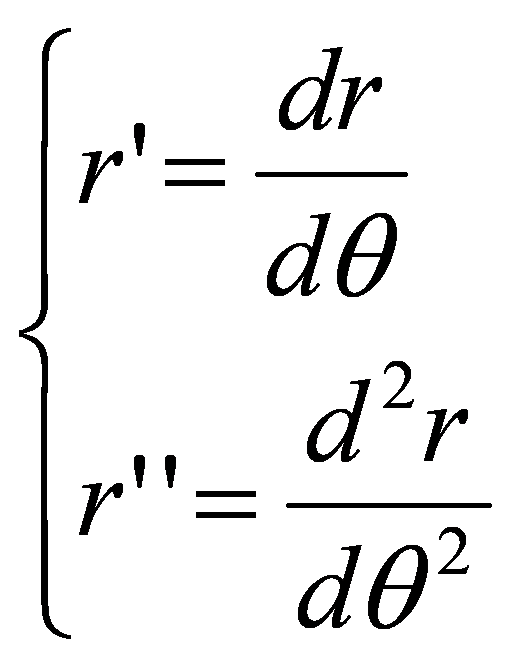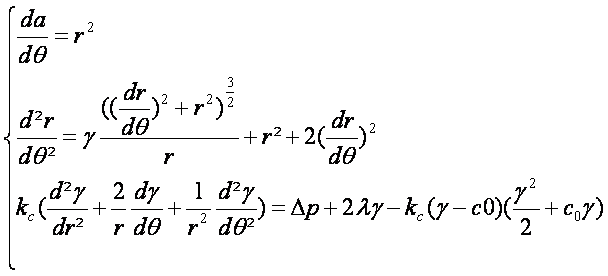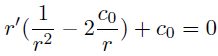Team:Paris/Production modeling2
From 2009.igem.org
(→"Periplasmic osmotic pressure increase") |
(→Unequal pressures create small blebbing:) |
||
| Line 75: | Line 75: | ||
| - | ::In the following we first consider a model without brownian movement of the Tol-Pal links that are considered immobilized and randomly | + | ::In the following we first consider a model without brownian movement of the Tol-Pal links that are considered immobilized and randomly distributed over the E.coli membrane. We look also here at a simplified one dimensional membrane. Our aim is to get a realistic visual representation of the equilibrium shape of the outer membrane for a given osmotic pressure difference. In one dimension and in polar coordinates the membrane equation of the membrane conformation given by Ou-Yang and Helfrich [Phys. Rev. Lett. 59 (1987) 2486] simplifies well. |
Revision as of 22:08, 20 October 2009
iGEM > Paris > DryLab > Vesicle biophysics Model (vesicle model)
Contents |
DryLab - Vesicles biophysics model
Pour Samuel et gregory
Unequal pressures create small blebbing:
Simplified approach: without brownian motion of the intermembrane Tol-Pal links
- In the following we first consider a model without brownian movement of the Tol-Pal links that are considered immobilized and randomly distributed over the E.coli membrane. We look also here at a simplified one dimensional membrane. Our aim is to get a realistic visual representation of the equilibrium shape of the outer membrane for a given osmotic pressure difference. In one dimension and in polar coordinates the membrane equation of the membrane conformation given by Ou-Yang and Helfrich [Phys. Rev. Lett. 59 (1987) 2486] simplifies well.
- Where H is the mean gaussian curvature and K is the Gaussian curvature :
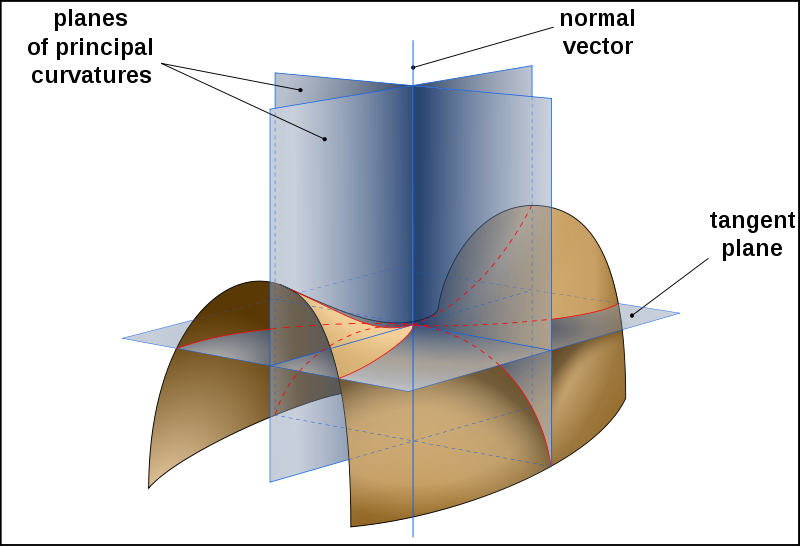
- The principal curvatures of a surface on the point M are defined as the minimum and the maximum curvature at this position of the curves described by cuting the surface with plans containing the normal direction at this point.
Principal curvature on a given surface [http://en.wikipedia.org/wiki/Principal_curvature#top| extracted from an article on Wikipedia]
- We first simplified the membrane equation to a 2 dimensional equation system based on a polar curvature simplification approximation :
- the curvature in polar is where
- Due to cylindrincal symetry the principal curvatures are always equal to the curvature in the perpendicular plane of the cylinder and the curvature in his axis so the mean and gaussian curvatures can be written as:
- indeed we have in those hypothesis:
- the osmotic pressure can be written in this way:
- And by concidering that we have low concentrations we can concider a simpliest formula analogue to the perfect gases law:
- Finaly we can write:
- Here we can see that the model is depending of the volume V which will stabelized the equation and define totaly the whole parameters of the model dynamic.
- With these relations the membrane equation becomes:
- In addition, we can translate the role of the Tol/Pal System as boundary condition on the whole system : cluster of Tol-Pal can be considered as a point with a radius equal to the peptidoglycan ones plus the length ot the protein. futhermore As the surface is closed, we must impose the fact that r(0)=r(2π) to account for closing the vesicle.
- the integration of the polar membrane equations gives results of the following type:
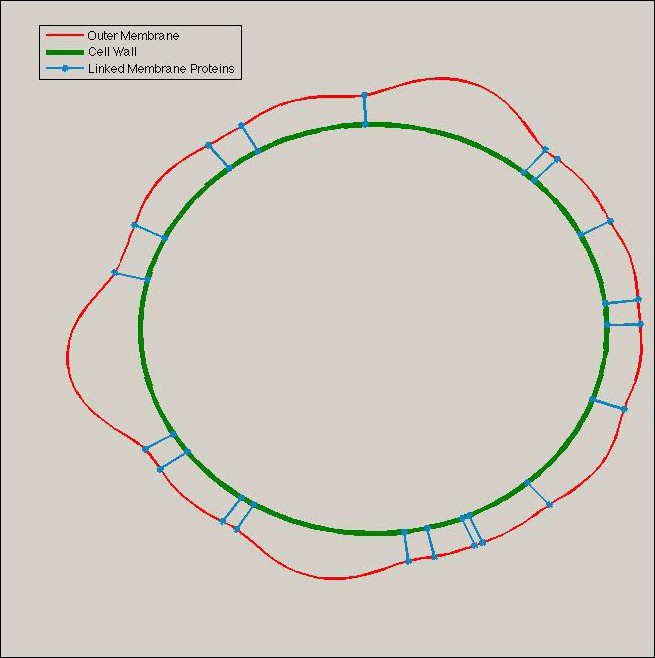
- Equilibrium shape of the outer membrane presenting blebbing (red) with immobile Tol-Pal links fixed to the inner membrane (green) assumed cylindrical obtain with an increase of the periplasm pressure.
The lipid surface conformation
- The Lipid conformation of the outer membrane is a well known problem: at 35°c the lipid bilayer behaves like a liquid which conformation character is ruled by an energy called the bending energy (R. Lipowsky, The Conformation of Membranes, Nature 349 (1991) 475-481). This energy represents the fact that the lipid bilayer will search a special conformation depending on the shape and the chemical properties of its constituents. In order to explain the way lipids organize together we need an expression for the membrane bending energy. This is given by :
- E is the energy of a whole lipid bilayer (or monolayer). Kb and Kg are Bending and Gaussian moduli which can be obtained by experiments. γ0 is the intrinsic curvature of the outer membrane which describes the local form of a lipid bilayer when it is at is lowest state of energy ,the more stable. γm and Hg are the mean curvature and the gaussian curvature( see Helfrich [Z. Naturforsch. C 28 (1973) 693] ). dS is a surface infinitesimal element and the previous formula relates how the local surface energy varies with the local mean curvature and the Gaussian curvature. Let us first calculate the energy of two different shapes of membranes, (i) a model of the shape of E. coli before budding, and (ii) after budding of a vesicle.
- (i) We consider the shape of E.coli as a cylinder of radius r =0.3.
- This is a simplified shape based on the heuristic fact that in septa where the vesicle will be constituted principally with lipids of higher bending energy. In fact this model is just a first approach to determine range of parameters and could be developed in a more sophisticated way later.The aim of this first representation is to estimate this energy in the division region of E.coli before division. With this approximation γm = 1/2r.
- Thus for the E.coli lipids membrane the bending energy per aera is:
- (ii) For the vesicles we consider their basic shape as a sphere of rayon r’ so the bending energy by lipid area units is:
- Thus as the area of a sphere is known and is independent of the location on the surface we can write:
- The energy of the same membrane area in E.coli is:
- The relations can provide a basic vision of the statistical repartition of vesicles in case of absence of integrity control system in the outer membrane.
- Ee is the potential energy of the lipid area in E.coli outer membrane before construction of a vesicle and Ev is the energy of the same lipid area but in the conformation after budding including a vesicle shape. So the energy which must be given to the whole system to create a vesicle is:
Mettre une courbe expliquant ceci
- We can suppose that the most easily created vesicles will be the ones which require a minimum energy. By derivation we find that the minimum is obtain for:
- Hence as we know that the range of created vesicles radii is 25 nm to 175nm we can suppose that the r’ is somehow about 100 nm. This leads us to an estimation of the outer membrane intrinsic curvature:
- Which is realistic in the order of magnitude
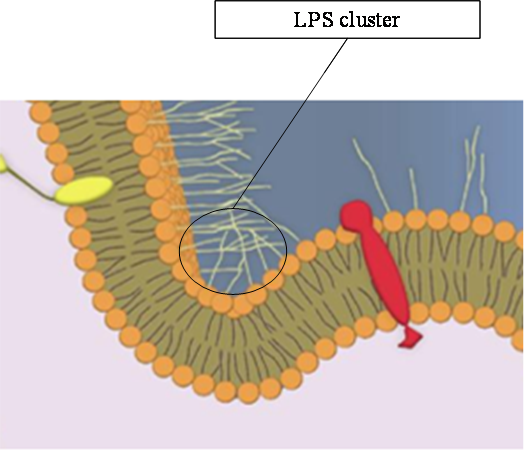
- In fact we know that the E.coli lipid bilayer is built of distinct types of lipids: Lippopolysacharides (LPS) and simple phospholipids. LPS are located in the exterior lipid layer of the outer membrane. The others are located in the interior lipid bilayer. Moreover, those LPS present a sugar extension toward the medium. Those sugars can bind to each other. So we can assume that they are going to create clusters and to curve the membrane toward the exterior of E.coli.
- This part was dedicated to a first approach of the membrane shape and its characteristics introducing some concept of lipid membrane physics. It enable us to find a range value for one important parameter.
Travail à faire
Reprendre pourquoi cette modélisation et introduire ce quel'on fait ici. Par ex. In this section we are going to develop an original biophysical model developed to explain the formation of vesicles. This study aims at a better understanding of the mechanisms leading to the production of vesicles by the cell a process still very poorly described [Références] despite the natural occurrence of this phenomenon in gram-negative bacteria. Knowledge of the underlying processes can prove valuable in the perspective of optimizing inter-cellular communication by vesicle transport.
ON PASSE DANS UNE AUTRE PARTIE: petite conclusion de ce qui précède et introduction de la suite
"Tol-Pal proteins diffusion"
Tol and Pal are membrane proteins which are located respectively in the outer and the inner membrane. The diffusion of proteins in those lipid bilayers can be modelled by a probabilistic Brownian movement. This diffusion model gives us the law of probability for the location of Tol and Pal on the membranes. It has been observed that the Tol and Pal proteins interact with each other, which is linked to the membrane stability: indeed the Tol and Pal will bind inner and outer membrane and furthermore stabilize the outer membrane using the peptidoglycan rigidity. FAIRE UN RENVOIT A LA PARTIE DU WIKI QUI PRESENTE LE SYSTEME TOL-PAL OU INCLURE ICI UN SCHEMA
Treatment including brownian motion of the intermembrane Tol-Pal links
We will now let the Tol-Pal links between the cell outer and inner membranes move freely with a brownian motion. This motion links the local concentration of proteins with the shape of the membrane they are included in. The diffusion of this links is described by:

|
where P is the proteins presence density of probability .
PIERRE, JE ME RAPPELLE QUE TOL ET PAL DIFFUSAIENT INDEPENDEMMENT ET POUVAIENT QUAND ILS SE RENCONTRAIENT FORMER DES LIENS. C'EST CE QUI SE PASSE ICI OU BIEN CE SONT DE LIENS TOL-PAL DEJA FORMES QUI DIFFUSENT? ETRE PLUS CLAIR ICI
The Laplacian of P explains the fact that the concentration of proteins like Pal increased in regions of negative curvature. Such a model can explain some recent observations [Kumaran & Losick] “negative membrane curvatures as a cue for sub-cellular localization of a bacterial protein”. According to this we can assume that the Pal will be confined to region with negative curvature. In those regions the probability of linking between Pal and Tol is increased which enhances the local membrane stability and contributes to enlarge these regions. PAS CLAIR POURQUOI LA VESICULE EST MURE.... Then the vesicle is mature.
::So Brownian motion is a good explanation for the proteins migration toward the septa during division. PAS CLAIR SI GARDER
- But this Brownian motion can explain the vesiculation too.
This reinforcement process driven by brownian motion can explain the vesiculation.When there are too few proteins we can assume the creation of a vesicle at germinal states (called bleb). At the border of the blebs an amount of Pal protein prevent the whole membrane to swallow and keep the vesicle in form. But at the border between the blebs and the protein rich area curvature is highly negative and so the quantity of proteins in those regions increases enlarging the region toward the centre of the blebs.
UN SCHEMA IMPERATIF
- Then two different options are expectable: the blebs is too small and then the proteins are “zippering” back the blebs. But if the bleb is big enough the zippering became a separation between the vesicle and the bacteria. The good vision of this blebs forming can be understood in this way: the proteins rich region on the border pass from an annular form toward a circular one.
 "
"