Team:Paris/Production overview Construction
From 2009.igem.org
iGEM > Paris > Vesicle production system > Constructions
Contents |
Construction
Creation of OMVs
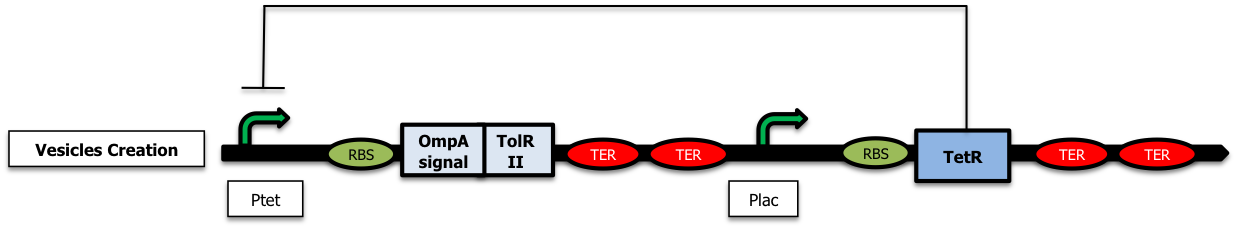
To create vesicles, we started with a first simple construction only containing the nonfunctional TolR II biobrick [http://partsregistry.org/wiki/index.php?title=Part:BBa_K257005 Bba K257005] fused with OmpA signal [http://partsregistry.org/wiki/index.php?title=Part:BBa_K257006 Bba_K257006] (which allows it to migrate in the periplasm) placed donwstream a ptet promoter (Freezer). In the presence of arabinose, the TetR repressor is expressed from the pLac promoter and inhibits the expression of tolRII (Figure a). The addition of tetracycline or of one of its analog, aTc, in the medium relieves the repression thus allowing the production of vesicles (Figure b).
For further precisions on the biobricks used as well as the plasmid backbones, please visit our parts design page .
The delay
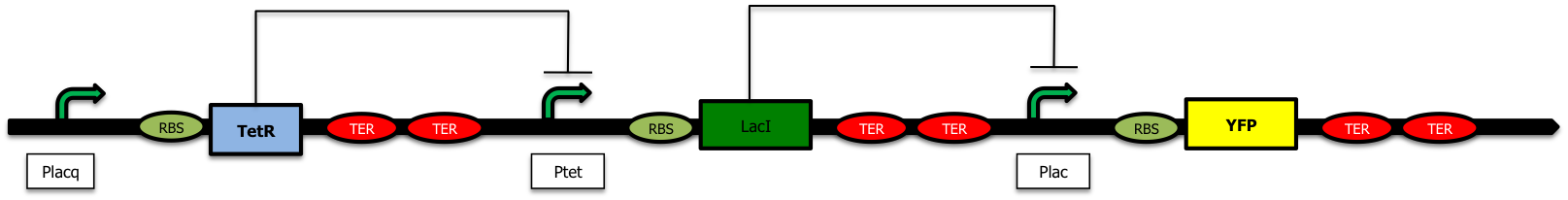
Our aim is to send vesicles containing proteins (the "message" in the bubble) into the medium and. To avoid a premature production of "empty" vesicles, we want to wait for the proteins to be present inside the periplasm or in the outer membrane before creaing vesicles. We have introduced a delay system inside the plasmids (Freezer). This kind of device was studied by Hooshangi & Weiss[1] where they studied a transcriptional cascade made of two repressor: TetR and LacI.
Nevertheless, as in this construction, the pLacI promoter is leaking, we decided to exchange the order of the two repressors to avoid a low but constant expression of TolR. When there is arabinose in the medium, the production of LacI represses the Plac promoter and prevents the creation of TetR; this way, the Ptet promoter is no longer repressed and GFP is expressed in the medium.
With this design, even if there is some leaky expression of the genes downstream the pLac promoter (when it is repressed), it leads to the creation of TetR and a stronger repression of the pTet promoter.
We also decided to place the pTet+TolRII device upstream the pLac+TetR cassette (Freezer); as a consequence, even if our first terminator is not a 100% efficient, the RNA-polymerase will transcribe the downstream part made of the TetR repressor. This repressor created by accident will then repress the expression of TolRII.
If we had switched these two parts, a bad efficiency of the terminator would have led to the expression of more TolRII which could be dangerous for the cell.
These considerations led us to construct this final system :
Notes :
- To get more information on the terminators we use, you can see the description in the registry for:
- [http://partsregistry.org/Part:BBa_B0014 BBa_B0014]
- [http://partsregistry.org/Part:BBa_B0015 BBa_B0015.]
- To read more on terminators, you can also have a look at the registry: [http://partsregistry.org/Help:Terminators Terminators.]
Caracterisation of the delay
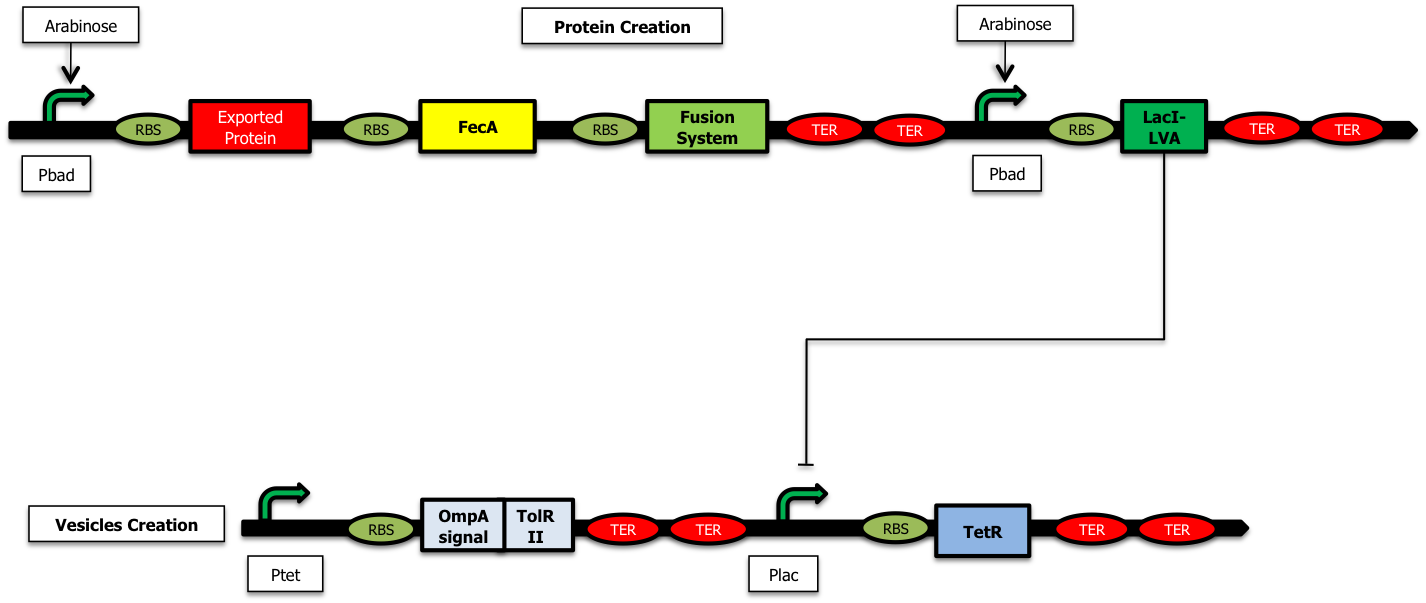
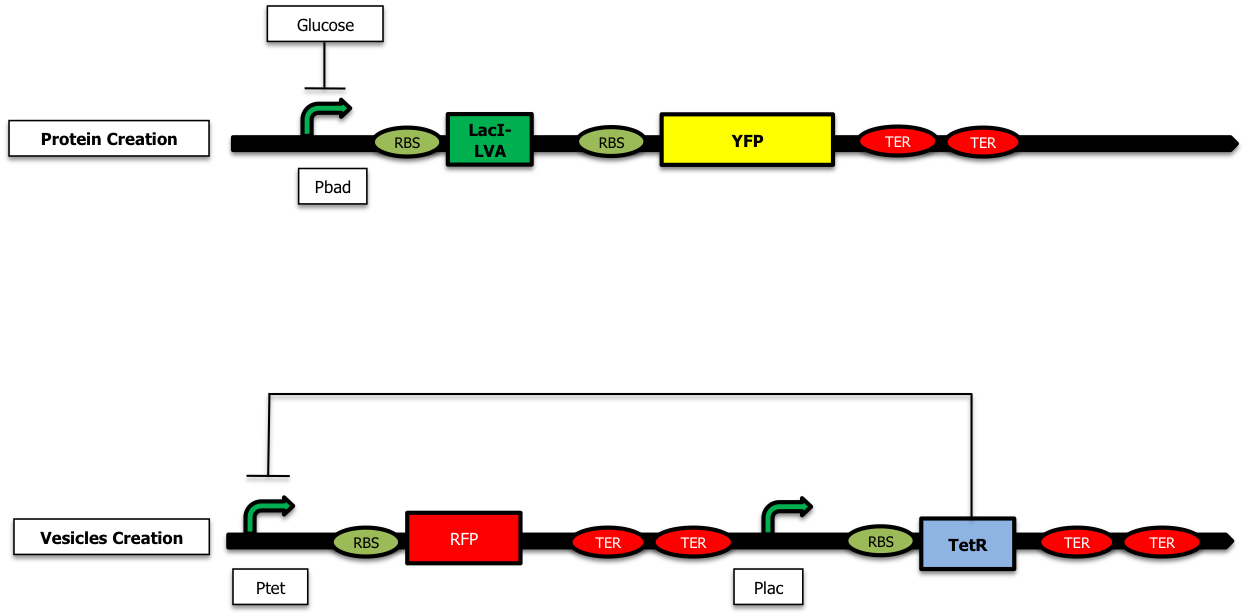
We have started the construction of a system which would allow us to characterize the delay created by our device. In this construction, instead of expressing the proteins we want to send inside the vesicules and the TolRII protein, we express the [http://partsregistry.org/Part:BBa_E0030 YFP] and the RFP respectively. Using fluorescent microscopy, we can try to measure this delay.
As shown on the figure below, once there is arabinose in the medium, the pBad promoter is ON allowing the creation of LacI ; LacI represses the pLac promoter and the TetR protein is no longer expressed. The pTet promoter is ON and RFP is created :
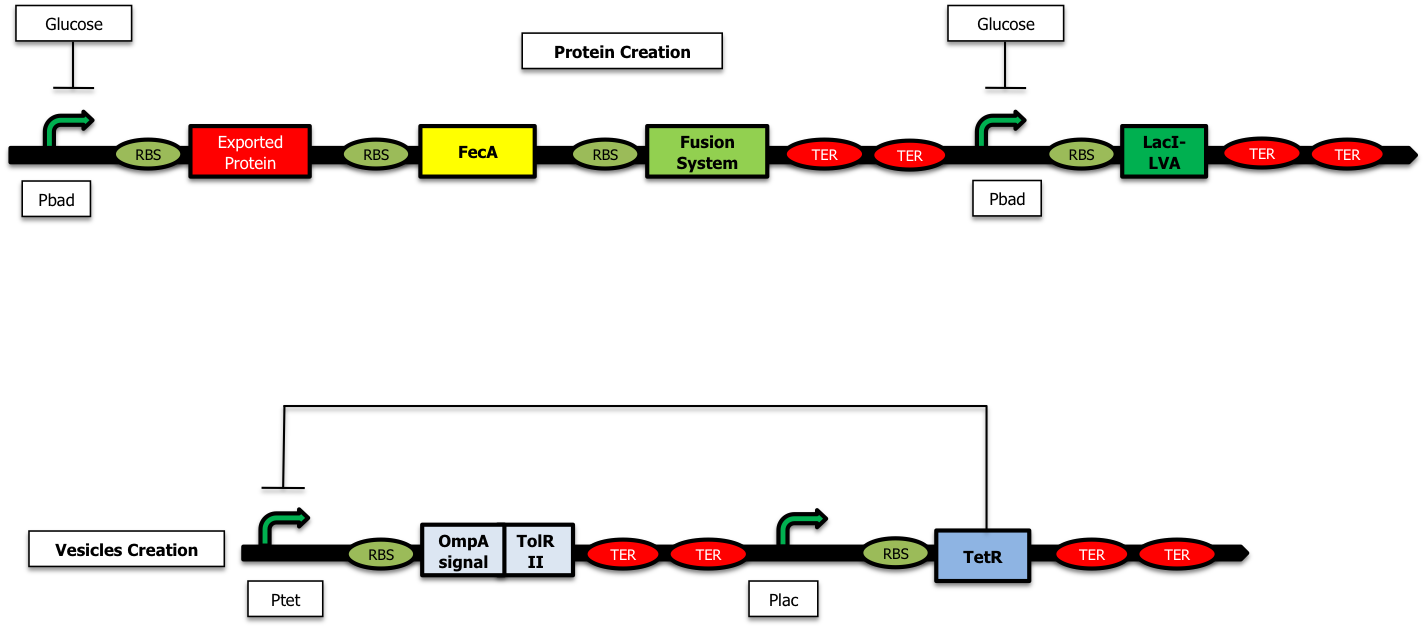
On the other hand, when there is glucose in the medium (and no Arabinose), LacI is no longer produced and the TetR protein is created : there is no more RFP synthetized :
The LVA tag
The LacI protein is supposed to be quite a stable protein in the cells ; as a way to reduce its half life and thus to decrease the strenght of the plac repression (see modelling part for analytic interpretation), we decided to use a LVA tag on the LacI protein.
The LVA tag is a short peptide sequence added to the C-terminal end of a nucleotide sequence ; in the periplasm, degradation of AANDENYALAA-tagged proteins is known to be the result of the Tsp protese whereas in the cytoplasm, it is due to a Tsp homolog. Mutations in the last three residues of the AANDENYALAA tail alter the protein stability[2].
These different mutations were studied by Andersen & Molin on the Gfp protein both in E.Coli and P.Putida. In both species, protein including a LVA mutation were the most rapidly degraded. These mutations were also used by Ellowitz and Leibler in the repressilator device in order to obtain an efficient oscillator.
References
- ^Ultrasensitivity and noise propagation in a synthetic transcriptional cascade S.Hooshangi & R.Weiss 2005 - [http://www.ncbi.nlm.nih.gov/pubmed/15738412 15738412]
- ^New Unstable Variants of Green Fluorescent Protein for Studies of Transient Gene Expression in Bacteria Jens Bo Andersen & Søren Molin 1998 - [http://www.ncbi.nlm.nih.gov:80/pmc/articles/PMC106306/ 106306]
 "
"