Team:Paris/Transduction modeling
From 2009.igem.org
iGEM > Paris > DryLa > Fec operon simulation
Contents |
DryLab - Fec simulation: Improving reception quality
As explained in the dry lab overview, the goal of this part is to answer the following question :
Indeed, our reception system uses the fec operon in order to transmit the signal received on the outer membrane via the vesicles into the cytoplasm and to induce a response of the receiver cells.
To have a reliable communication system, it is crucial to have a robust response despite a potentially very low number of signals (ie, vesicles) received. Because we typically assume that few messenger molecules will be present, we performed stochastic simulations to investigate this problem.
Modeling the reception system: Fec operon
In order to get a good activation of the receiver cells despite a potentially very low number of messenger molecules, our design is based on the over-expression of constitutively active molecules that induce a positive retro-action.
The chemical equations
Transduction of signal is initiated by FecA molecules coming from the vesicles. When these molecules reach the bacterial outer membrane, FecR proteins are activated. This extracellular signal transduction from OMV to the outer membrane is represented by the following reaction :
Once activated, the FecA molecules are able to activate the FecR proteins constitutively present in receiver cells.
In the following work, we assumed that this step, corresponding to the crossing of the periplasm is the limiting step. Consequently, we decided to distinguish two ways to describe this step:
- No complexation: the FecA molecule directly activates FecR, and the phenomenon is described in a single reaction :
- Complexation: FecA and FecR form a complex which is then dissociated to release an activated FecR protein. This mechanism is described through the 2 following reactions :
The rest of the signal transduction pathway is described through a series of reactions listed below and shown on the diagram. To get more details on this description, please see our ????? page.
In the above diagram, FecR_Nterm corresponds to the N-terminal part of the FecR protein which is then constitutively active: the FecR_Nterm is able to activate FecI without the need of FecA. This is the main component of the positive retro-action loop.
Gillespie algorithm
We used mass action laws to model the reactions. Each reaction has a kinetic constant k determining its reaction rate, given the concentration of the reactants. The constant names corresponding to each reaction are listed here.
As the number of molecules at stake can be very low at the beginning of the reception, we performed stochastic simulations based on the Gillespie algorithm to study the properties of our system [1][2][3]
Stochastic simulations
As explained above, the crossing of the periplasm is considered as the limiting step of the signal transduction pathway. Therefore, we performed various runs of simulations using different values of the kinetic constant k_2 characterizing the activation of FecR by FecA across the periplasm.
The results of these runs are gathered in the table below showing the quantity of activated cells for the two modeling assumptions we considered:
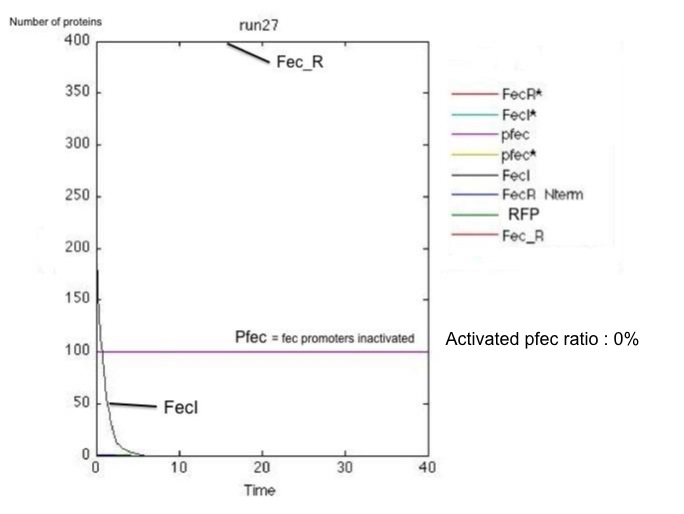
These results and the examination of the plots below showing the activation of the pfec operon in receptor cells highlighted two major problems:
- the reception system is poorly robust : for little amounts of FecA, the activation occurs only in half of the cases.
- the time of activation varies significantly from one cell to the other
These problems are encountered under both modeling hypothesis (complexation / non-complexation).
Non-robust response for little amount of FecA and poorly efficient FecR activation (k_2 small): on the left, the receiving cell is activated, and the RFP produced downstream of the pfec promoter has reached a steady state level; on the right, the vesicles have failed to activate the transduction cascade and the fec operon.
Significant variations of the activation times: on the left, the activation time is quite long compared to the situation on the right (about 4 times greater).
Given these simulations results, we calculated the mean and standard deviation of the activation time:
- No Complexation, little amount of FecA, poorly efficient FecR activation (k_2 small)
- Complexation, little amount of FecA, poorly efficient FecR activation (k_2 small)
These values confirmed the significant variability of response times.
Getting a robust reception
To overcome this variability we tried to figure out a way to make the crossing of periplasm easier ; among the solutions that we thought of, the over expression of FecR molecule in receiving cells seemed to be the most appropriate. The presence of a greater number of FecR increases the probability of interaction with FecA molecules coming from vesicules.
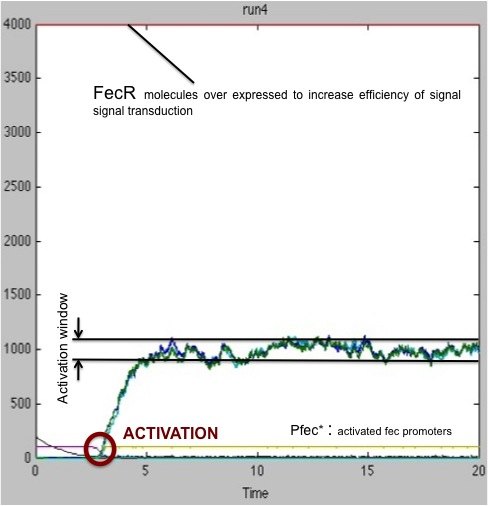
We ran another set of simulations, this time only focusing on the case that had given bad results in the previous study ; this time, the quantity of FecR constitutively present in the cells was raised from 400 units to 4000 units.
As seen on these results, the presence of a greater amount of FecR allows a better transduction of signal received. In 100% of cases, the receiving cell is activated. However, the activation time is still fluctuating significantly.
Therefore, our advice to the biologist would be to express FecR protein in receiving cells to ensure a robust and reliable reception of signal.
References
- ^Exact Stochastic Simlation of Coupled Chemical Equations. Gillespie Daniel T. 1977 - [http://www.dna.caltech.edu/courses/cs191/paperscs191/gillespie2.pdf Gillespie1]
- ^The Chemical Langevin Equation Gillespie Daniel T. 1997 - [http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=JCPSA6000113000001000297000001&idtype=cvips&gifs=yes Gillespie2]
- ^Logarithmic Direct Method for Discrete Stochastic Simulation of Chemically Reacting Systems H.Li & L.Petzold 2006 - [http://www.cs.ucsb.edu/~cse/Files/ldm0513.pdf Sto.Sim]
draft
Our reception system uses the fec operon in order to transmit the signal received on the outer membrane via the vesicules into the cytoplasm to induce a response of the reception cells. Once the vesicule has fusioned with the cell, the FecA molecule present on the previous vesicule wall activates the FecR molecule on the inner membrane ; then, the FecI molecule is activated and the pFec promoter is ON and transcription can start.
The plasmid of this reception cell was designed to produce a positive reaction once the signal is received so that, even with a very faint signal (very very few FecA molecules inside the vesicules), the reception population will be activated. To this end, we have placed the of FecR and the FecI coding downstream th epfec promoter. This way, once the first FecR and FecI molecules are produced, they take part in the activation of the pfec promoter thus creating a positive retroaction.
Our modelling study focussed on the description and analysis of this trancriptional cascade in order to caracterize the ability of the system to receive a signal. To this end, we tried to describe the system with elementary chemical equations allowing both deterministic and stochastic simulation runs.
To get more information on this study, please visit the following sections :
- The Fec Operon in our system to read more about the chemical equations chosen to describe the system and the kinetics equations associated
- Deterministic and Stochastic Simulations to read more about the different simulations.
- Results and discussion to learn more about the results of this study.
 "
"