Team:SJTU-BioX-Shanghai/Characterization
From 2009.igem.org

Project introduction. Inspired by the natural regulator of circadian bioclock exhibited in most eukaryotic organisms, our team has designed an E.coli-based genetic network with the toxin-antitoxin system so that the bacterium oscillates between two states of dormancy and activity (more...)
Part characterization
Characterization of RelE toxin+Double terminater([http://partsregistry.org/wiki/index.php?title=Part:BBa_K185004 BBa_K185004])
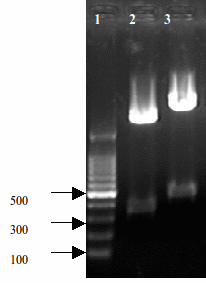
It is a compsite part composed by BBa_K185047 and BBa_B0015. The creator of this part is Alex Jiang. When we insert different promoter ahead of this part, it will express RelE protein on different levels, depending on the strength of promoters. Its length is 443 bp, which can be verified by gel electrophoresis.
- Fig 1. Construct results of RelE+Double terminator
- Lane2: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K185000 BBa_K185000] RelE toxin+Rbs30 311bp
- Lane3: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K185004 BBa_K185004] RelE toxin+Double terminator 443bp
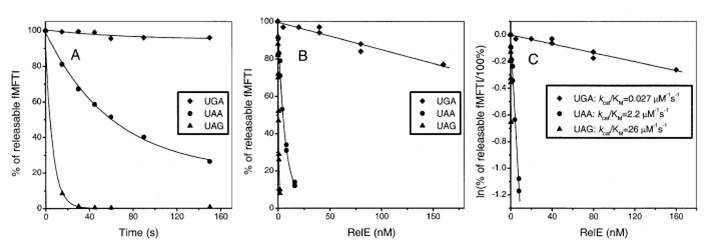
The stop codon of wild type RelE gene is TGA. Here we mutated it into TAA, according to inhibition specifity towards different stop codons: UAG>UAA>UGA. The rates of the inhibition reactions in all cases were proportional to the relE concentration and thus determined by kcat/Km. The kcat/Km Values (s-1μM-1) for RelE Cleavage of 3 stop codons in the Ribosomal A Site have been measured previously. (UGA:0.078, UAA:2.2, UAG:26).
We have test this part under two different promoters: pLac and pBAD. After identification of reconstruced plasmid, we transfected the plasmid into E.coli BL21 strain and then incubated it into M9 medium. All the liquid M9 medium contains 0.1% IPTG and 0.1% Amp(In pBAD strain, 0.2% L-arabinose and 0.1% Amp), in order to keep the same growing conditions. The total amount of bateria is detected by spectrophotometer. It provides the real-time detecting data of bacteria amount under OD600.
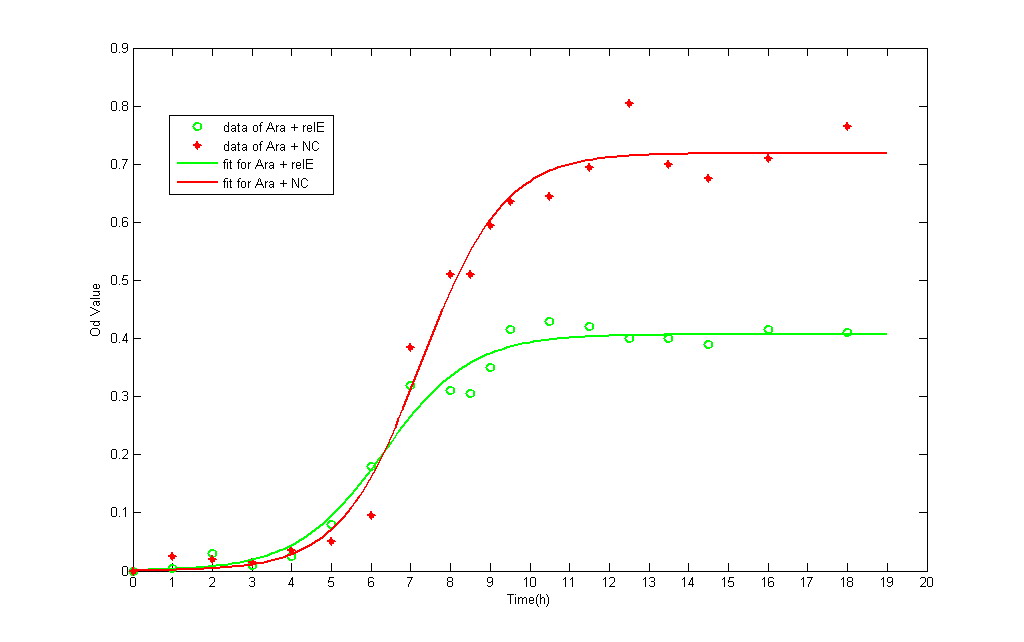
The growth data and fitting curve (according to logistic formulation) are demonstrated below:
- Table 1 Variation of OD600 Value with Time in Different Strains
Time(h) OD(pLac+RelE) OD(NC in IPTG) OD(pBAD+RelE) OD(NC in Arabinose) 0.000 0.0000 0.0000 0.0000 0.0000 1.000 0.0250 0.0100 0.0050 0.0250 2.000 0.0500 0.0250 0.0300 0.0200 3.000 0.0050 0.0100 0.0100 0.0150 4.250 0.0000 0.0150 0.0250 0.0350 5.000 0.0500 0.0100 0.0800 0.0500 6.000 0.1400 0.0350 0.1800 0.0950 7.000 0.2350 0.1500 0.3200 0.3850 8.000 0.2650 0.3350 0.3100 0.5100 8.500 0.2550 0.3150 0.3050 0.5100 9.000 0.2500 0.3250 0.3500 0.5950 9.500 0.2300 0.3600 0.4150 0.6350 10.500 0.3000 0.4150 0.4300 0.6450 11.500 0.3300 0.4550 0.4200 0.6950 12.500 0.2950 0.4900 0.4000 0.8050 13.500 0.2750 0.4800 0.4000 0.7000 14.500 0.2500 0.4600 0.3900 0.6750 16.000 0.2550 0.5050 0.4150 0.7100 18.000 0.2400 0.4700 0.4100 0.7650
- The green line represents the OD value of NC, whereas the red one represents RelE strain.
- The green line represents the OD value of NC, whereas the red one represents RelE strain.
The growth curve of both NC and RelE strain fit the typical bacteria growth pattern, including the lag phase, exponential phase, stationary phase, and death phase. However, the exponential phase (the most rapid phase in which bateria grows) in RelE strain shows a shorter period than in NC group. The shorter exponential period eventually results in lower concentration of bacteria in stationary phase. Consequensely, we can verify that expression of the relE gene has been shown to severely inhibit bacteria growth and prevent colony formation.
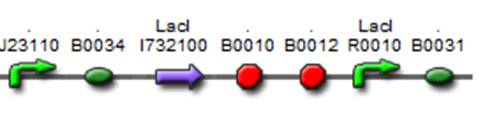
Characterization of an inverter of lac system([http://partsregistry.org/wiki/index.php?title=Part:BBa_K185033 BBa_K185033])
It is an inverter which its downsteam gene will always be repressed by lacI protein unless IPTG is added. And the synthetic rate of the downsteam gene is low because the low binding efficiency of B0031.
- And I choose to assemble [http
- //partsregistry.org/wiki/index.php?title=Part:BBa_I712019 BBa_I712019] with it. Here is the data:
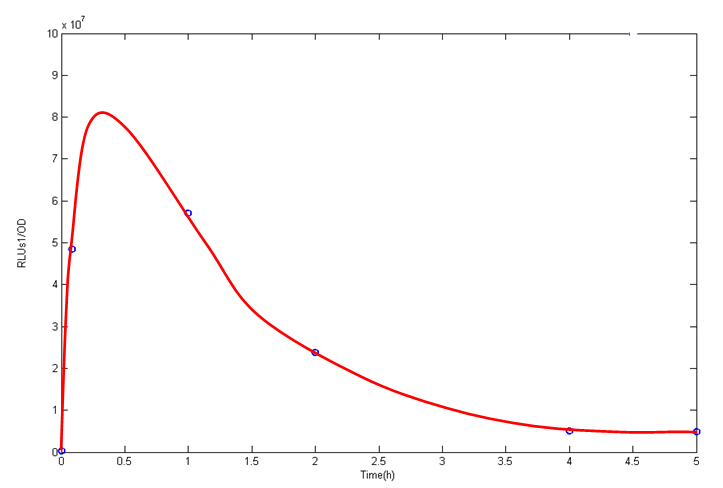
Sample OD RLUs1 RLUs1/OD A1 2.64 8090131 3064444 A2 1.83 12033165 6575500 B1 2.74 14369947 5244506 B2 1.67 8374294 5014547 C1 1.79 34906168 19500653 C2 1.58 44358500 28075000 D1 0.7 59173888 84534126 D2 0.89 26208740 29448022 E 0.54 26208740 48534704 F 1.45 383217 264287.6
- Sample A1/A2 are BL21 strain carrying BBa_K185033, induced by IPTG. for 5h.
- Sample B1/B2 are BL21 strain carrying BBa_K185033, induced by IPTG. for 4h.
- Sample C1/C2 are BL21 strain carrying BBa_K185033, induced by IPTG. for 2h.
- Sample D1/D2 are BL21 strain carrying BBa_K185033, induced by IPTG. for 1h.
- Sample E is BL21 strain carrying BBa_K185033, induced by IPTG. for 5min.
- Sample F is BL21 strain carrying BBa_K185033, but it is not induced by IPTG.
From the data above, we know that the downstream genes (luciferase) are switched on sharply (about 100 times the uninduced sample) in the initial 1 hour. And then the synthesis of luciferase decreases into about 10 times the uninduced sample. It is certificated that our lac system is working.
Here is the data for Fig 3 & Fig 4: SJTU09_data_for_3_pictures.zip.
Next, go to Human practice of our project.
 "
"