Team:Sheffield/Modeling
From 2009.igem.org
Kirubhakaran (Talk | contribs) |
Kirubhakaran (Talk | contribs) |
||
| (One intermediate revision not shown) | |||
| Line 8: | Line 8: | ||
!align="center"|[[Team:Sheffield/Team|Team]] | !align="center"|[[Team:Sheffield/Team|Team]] | ||
!align="center"|[[Team:Sheffield/Project|Project]] | !align="center"|[[Team:Sheffield/Project|Project]] | ||
| - | !align="center"|[[Team:Sheffield/ | + | !align="center"|[[Team:Sheffield/Further Work|Further Work]] |
!align="center"|[[Team:Sheffield/Modeling|Modeling]] | !align="center"|[[Team:Sheffield/Modeling|Modeling]] | ||
!align="center"|[[Team:Sheffield/Notebook|Notebook]] | !align="center"|[[Team:Sheffield/Notebook|Notebook]] | ||
| Line 140: | Line 140: | ||
| + | |||
| + | |||
| + | |||
| + | ===General description=== | ||
| Line 155: | Line 159: | ||
Where y is activity of LacZ (miller units) and t is time (hours) | Where y is activity of LacZ (miller units) and t is time (hours) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Latest revision as of 16:21, 18 October 2009
| Home | Team | Project | Further Work | Modeling | Notebook |
|---|
|
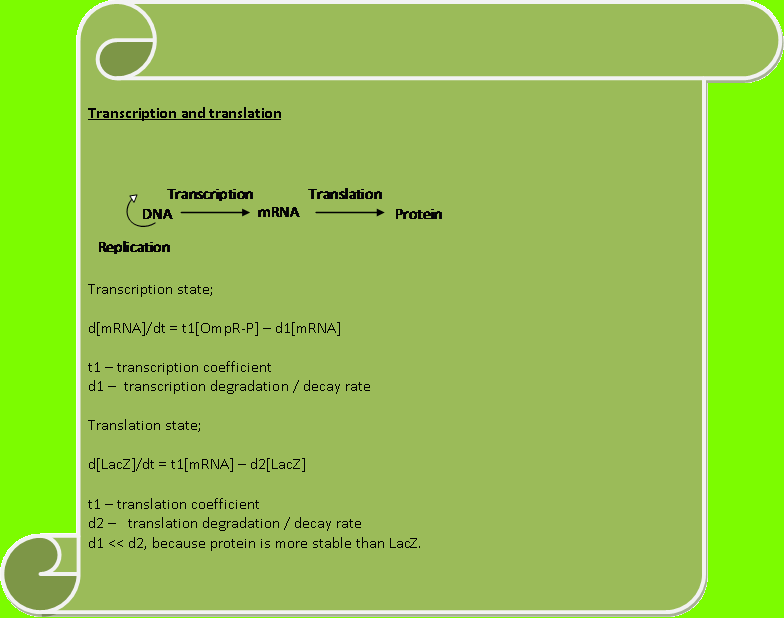
SYSTEM BREAKDOWNWe are going to analyse the biological system in a control perspective way. Our 3 main aims are; 1) Suggesting a realistic model for the system 2) Comparing the wet lab result with our model 3) A standard description of how our overall system intends to work.
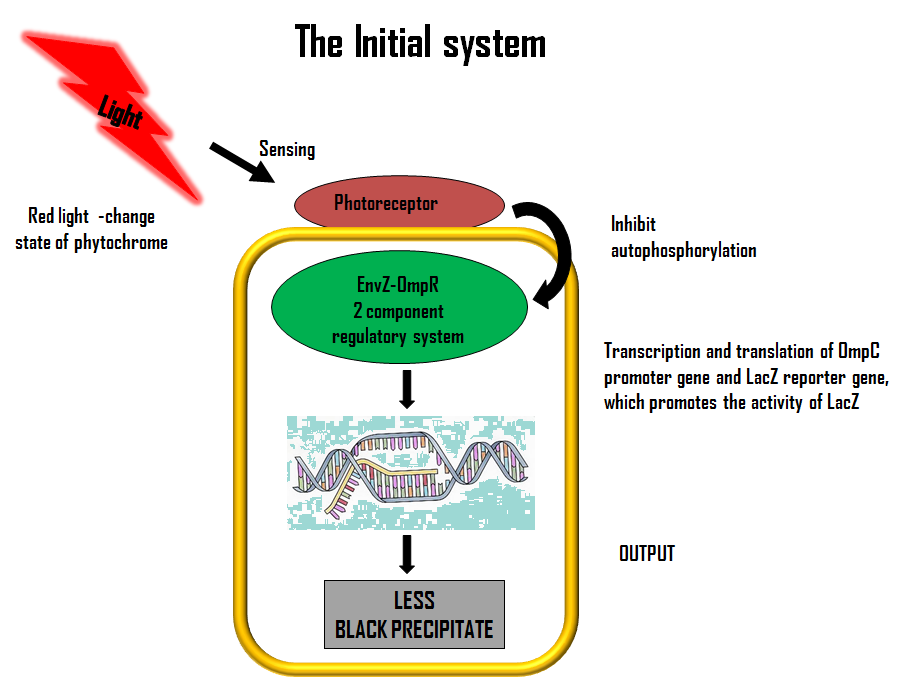
ModelLets first look at how our initial system works. The flow diagram below gives a brief description of it;
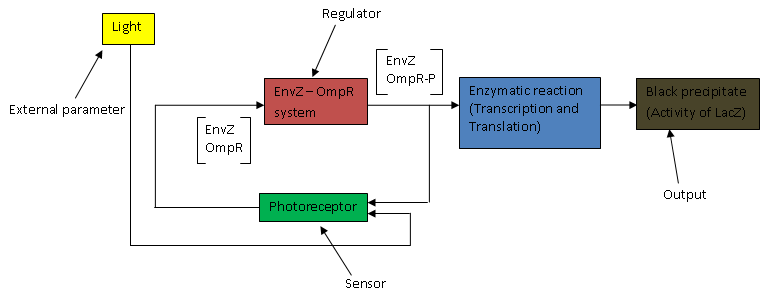
The above flow chart can be reconstructed into a control block diagram;
The script below shows how each block parameters are designed
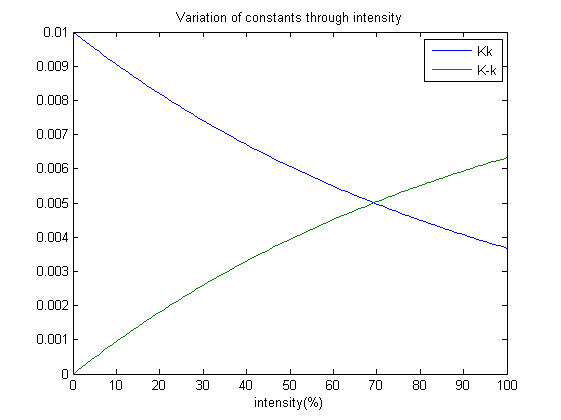
Our next step is to simulate the model, which brings about an interesting design practise which my team has employed. Which is, how does the photoreceptor works? And how does it affect the system when light intensity varies? A point to note; each ODE’s tends to a steady state after some time. Therefore we have suggested that: Constants Kk and K-k varies with the amount of light shined on the system and as well achieves a steady state at a certain point. Without the presence of red light, the concentration of OmpRP should increase. Therefore the constant Kk which affects the rate of reaction should decrease with intensity. Whereas K-k should increase as intensity increases.
k1=0.01, k-1=0.01, k2=0.01, k-2=0.01, kp=0.01, kt=0.01.
Initial concentration of: EnvZ = 1M, EnvZP = 1M, (EnvZP)OmpR = 1M, OmpRP = 1M, OmpR = 1M, EnvZ(OmpRP) = 1M.
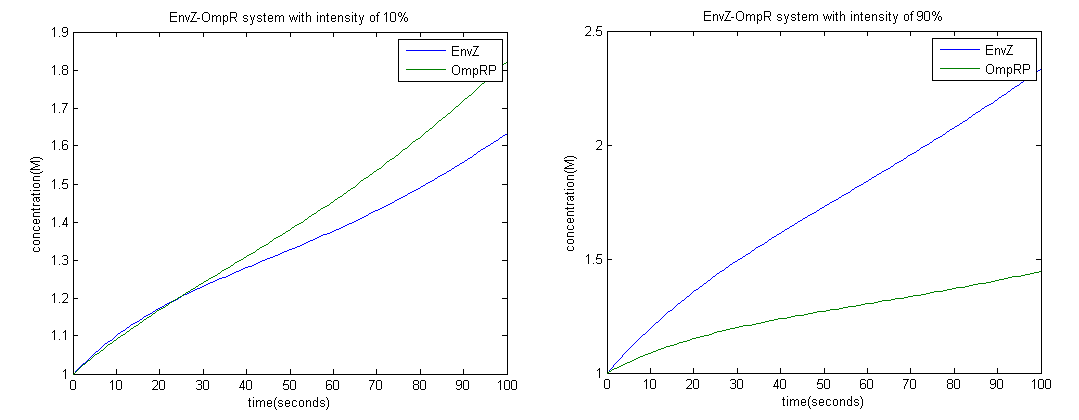
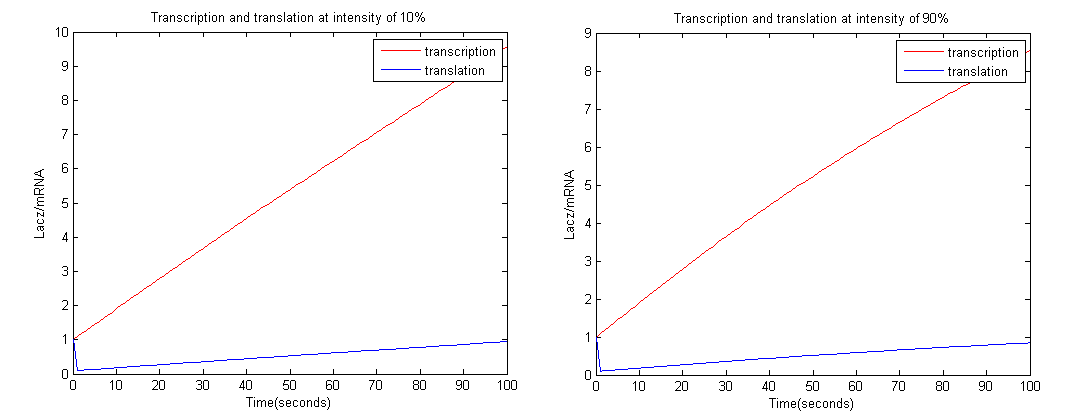
To achieve this we employed the Euler method; with a time interval of 1 second.
From the graph above it is clear that at high intensity OmpRP concentration is less, therefore activity of LacZ should be less as well.
Constants used: t1=0.1, t2=0.1, d1=0.01, d2=1.
From the graphs above we could see that at intensity 10% the amount of LacZ activity tends to 1 more than 90%. Therefore more black precipitate is formed at 10% rather than 90%, which proves the model.
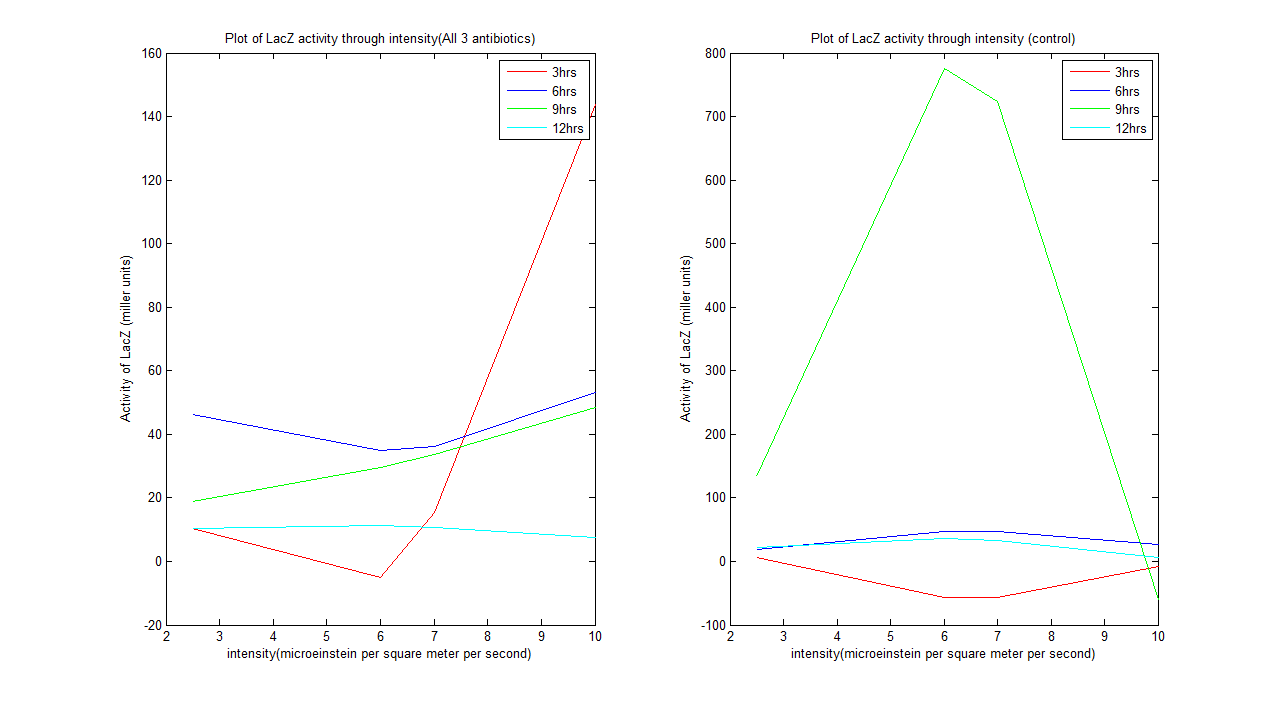
Wet lab result analysis
From the graph above we could see that the system does behave as our predicted model after 12 hours. The system tends to be unstable before it. A kinetic model can be defined for the system after 12 hours, which is: For all 3 antibiotics y= -0.1518x^2 + 1.5289x + 7.5641 For control y= -1.5133x^2 + 16.8821x - 11.1247 Where y is activity of LacZ (miller units) and x is intensity (microeinstein/square meter/ second)
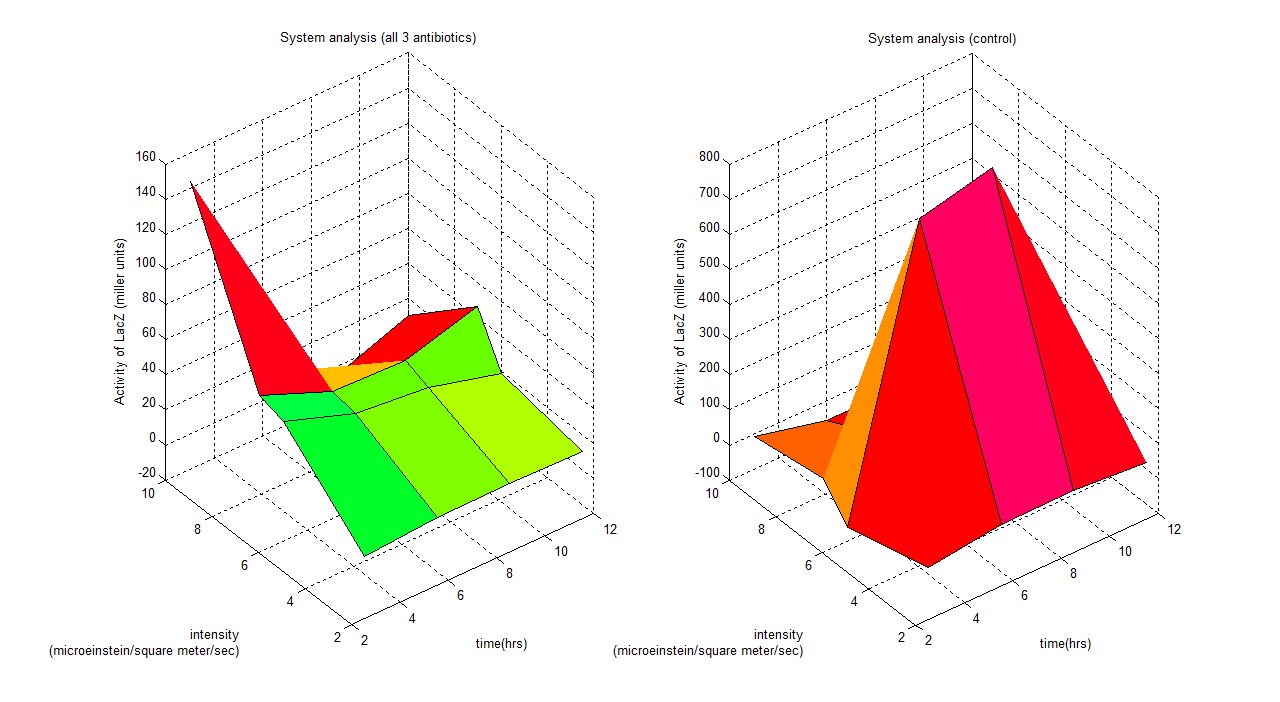
General description
These graphs show a clear idea of how the whole system binds together, which has 3 parameters varying (intensity, time and activity of LacZ). Any design process which involves this system could use this to predict the outcome of the model. For instance if we are working with this system under a wavelength of 6 (all 3 antibiotics), a system identification process could be employed showing a time dependent model;
Where y is activity of LacZ (miller units) and t is time (hours)
References[http://www.pnas.org/content/100/2/691.full# 1) Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system(Eric Batchelor and Mark Goulian)]
|
 "
"