Team:SupBiotech-Paris/Concept2
From 2009.igem.org
Contents |
Cell vector
The cell vector has to reach the target cell and delivers the therapeutic gene insert.
To be an efficient targeting cell vector, he has to get several important characteristics. He has to target specifically the interest cell type, the one to cure. As a phage vector, he has to be able to cross the eukaryotic cell membrane and deliver his content in an efficient way.
You are going to discover, across this chapter, what kind of characteristics have been decisive in order to choose the best bacteriophage.
Then, you are going to discover, in details, its properties according to the DVS characteristics: tissue deliverance, therapeutic plasmid encapsidation, and cell targeting.
The cell vector: The lambda phage [1,2]
We have studied several kind of phages, in order to select the cell vector the most controllable, and adaptable. So, we have identified all the different kind of phages, and in one kind of phage, the species the most described. Several candidates have retained our attention, but it is the lambda phage which seems to be the most adapted.
The lambda phage is a prokaryotic virus, which infects the bacteria Escherichia coli. This is a tempered phage, because it can alternate lytic and lysogenic cycle under some conditions. During the lytic cycle, the circular DNA is replicated in great quantity in the cytoplasm; a lot of viral progeny are formed, driving to the cell death and the virions liberation. During the lysogenic cycle, the phage genome is integrated in the bacterial chromosome, and is transmitted to the line of descent. The replication is achieved at the rythm of the bacterial cell division.
Naturally infectious for the bacteria Escherichia coli, the lambda phage, as every phage, is not infectious for man. This characteristic comes from the fact that the genome is governed by prokaryotic promoters but also because it has a very low capacity to transfect eukaryotic cells.
Finally, one of the most interesting characteristics of the lambda phage is the fact that he has a linear double stranded DNA, with cohesive ends: 12 nucleotides complementary “sticky” ends, called “Cos” sequence. These cohesive ends have a genomic encapsidation function.
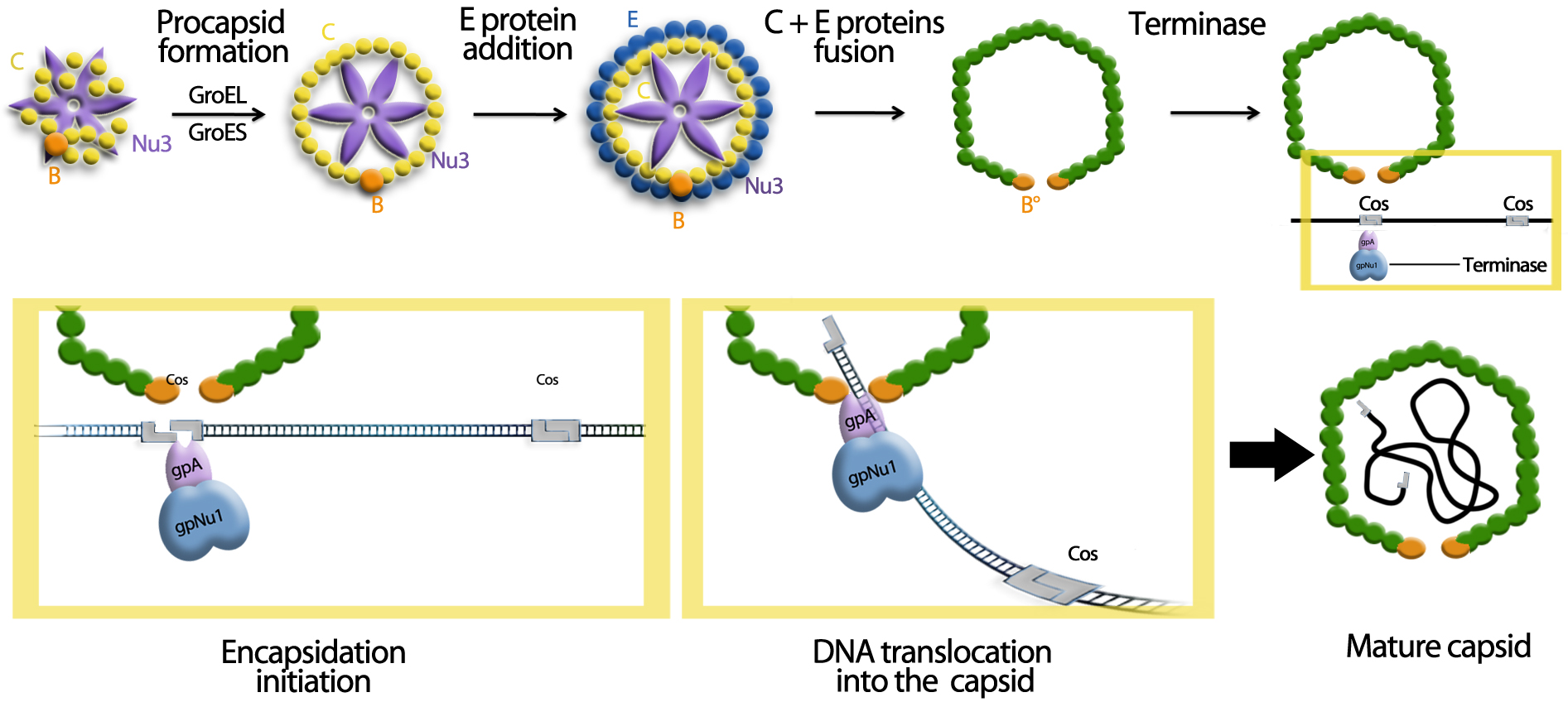
The encapsidation of the therapeutic plasmid [2,3,4,5,6,7]
The encapsidation of the viral genome is particular for the lambda phage. It is managed by the Cos sequence. This characteristic allows the specific encapsidation of the sequence of interest.
We can observe in this picture that the initiation and the terminaison of the encapsidation is mediated by the Cos sequence. This is the fragment between two Cos sequences which is encapsidated. This particularity is used in molecular biology since several years in order to create DNA banks and molecular tools named Cosmides.
According to this fact, we decided to encapsidate, inside the cell vector, only the therapeutic plasmid. So, the Cos sequence of the lambda genome is deleted, and are placed in the therapeutic plasmid.
Non-coding sequence Cos: Position 1-132 et 48462-48502 – length: 174 bp.
1.....TATCACTTTACGGGTCCTTTCCGGTGATCCGACAGGTTAC
41....GGGGCGGCGACCTCGCGGGTTTTCGCTATTTATGAAAATT
81....TTCCGGTTTAAGGCGTTTCCGTTCTTCTTCGTCATAACTT
121...AATGTTTTTATTTAAAATACCCTCTGAAAAGAAAGGAAAC
161...GACAGGTGCTGAA
The cell vector encapsulates the therapeutic plasmid inside its capsid because of the Cos sequence. Thus, it can deliver the therapeutic agent in the cells, which allows a supplementary precaution, in case of the interest vector is not inside the target organ.
The cell vector deliverance in the target organ [1,2,8]
The cell vector has two actions phase, a latency phase and an expression and release phase. During the latency phase, called lysogenic cycle, the genome stays “inactive” and no cell vector is produced inside the tissue vector. Whereas, during the expression and release phase, called lytic cycle, the genome is transcribed and replicated, in order to produce the cell vectors.
However, the tissue vector is not the natural host of the cell vector. So, if we want to induce a latency phase, we have to cause it. The deliverance system introduced into the tissue vector is designed to induce this latency phase.
The cell vector plasmid of the deliverance control induces a lysogenic cycle of this one.
The lysogenic cycle is a phenomenon naturally present in the lambda phage, the cell vector. During this cycle, the viral genome is inserted into the bacterial genome as a prophage, where every transcription is repressed. Only the cI gene, responsible of the lysogenic cycle maintain, is expressed.
The cI fixation on the operators under the control of pR and pL promoters, inhibited the genes expression and allows the lysogeny maintain. cI inhibited also its own promoter, pRM, allowing an autoregulation of its transcription.
The phage is maintained under the lysogenic form as long as cI is expressed. In the case of the DVS, the expression of cI is not controlled by the pRM promoter, but induced by the repressible promoter LacP/O fused to the cI gene. If this one is not repressed, the gene expression is inhibited, as the lambda phage natural pathway.
But, as soon as the deliverance signal of the cell vector, the doxycyclin, is injected, the LacI repressor, synthesized by the tissue vector, represses the repressible promoter LacP/O. cI inhibited, the genes can be transcribed, and the cell vector synthesized.
System without doxycyclin:

As soon as the cell vector is synthesized and released, the cell vectors lyse the tissue vector. This is an advantage, because it allows the destruction of the potentially pathogen agent of the DVS.
Moreover, no sooner, the cell vector is released in the target tissue, the cell vector targets the interest cells in order to deliver the therapeutic plasmid.
The system of cell targeting [1,2,9]
The cell vector is used in order to target the interest cells. The adding of cell targeting proteins, inside the lambda phage genome, is necessary.
The deliverance of the therapeutic plasmid is achieved in two steps: the cell targeting and the cytoplasmic release.
The interest cell targeting
The lambda phage naturally interacts with its target cell, Escherichia coli, via its tail fiber protein J. Only the protein J mediates the specific recognition of its bacterial host. It is, consequently, the ideal candidate to a modification which allows a change in the natural tropism of the phage.
In order to do this, the protein J is fused to a protein, which is able to bind specifically to a targeted cell receptor. The protein J interacts with the Escherichia coli membrane receptor by the Cter end that is why, we decided to bind a fusion protein to this side.
The protein J gene is fused at the 5’ end to the targeting protein gene. The protein which results of this construction, naturally transcribed during the cell vector synthesis, is expressed with the same frequency as the native protein. So, during the self-assembly, the cell vector has a targeting protein on his tail.
According to the targeted cell type, the ligand can change, but the location, next the protein J, stays the same. The DVS is consequently adaptable to several pathologies.
The internalization of the cell vector and the release of the therapeutic plasmid
The protein fused to the J protein mediates the targeting of the cell vector, but, for the internalization and the endosomal escape, the fixation of a membrane ligand is not sufficient. That is why, the cell vector have a second recombinant protein. This one is fused to another protein, not on the tail but on the head of the phage, the protein D.
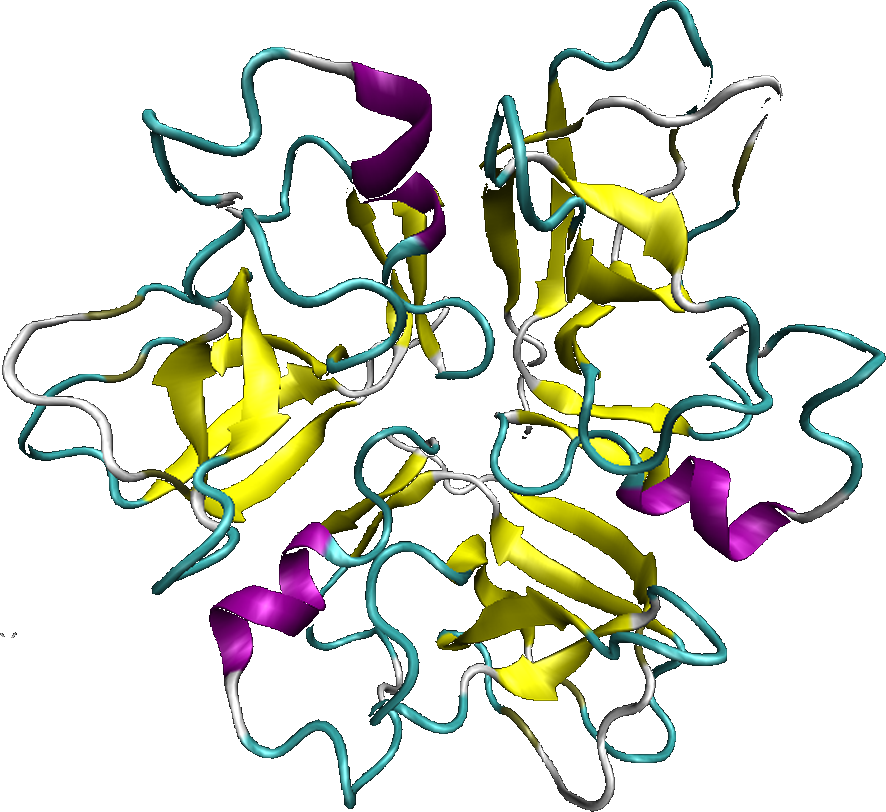
The lambda phage wild type has on the capsid, the protein D, which is in charge of the stabilization of the entire capsid, as we saw previously. The protein D is exposed on the entire outer surface of the capsid, that is why, it is an ideal candidate to be fused to a cellular internalization protein.
The D protein is organized as a trimer. Each monomer is distant of 5,1nm in a trimer. The crystal structure of the protein D, as a trimeric form, shows that the Nter and Cter ends, are exposed at the capsid surface, and are very closed from each other. The Nter and Cter ends are exposed at the mature capsid surface, which allows the choice for the location of the fusion protein (Nter or Cter). However, several studies have showed that the fusion to the Cter end, allows better results.
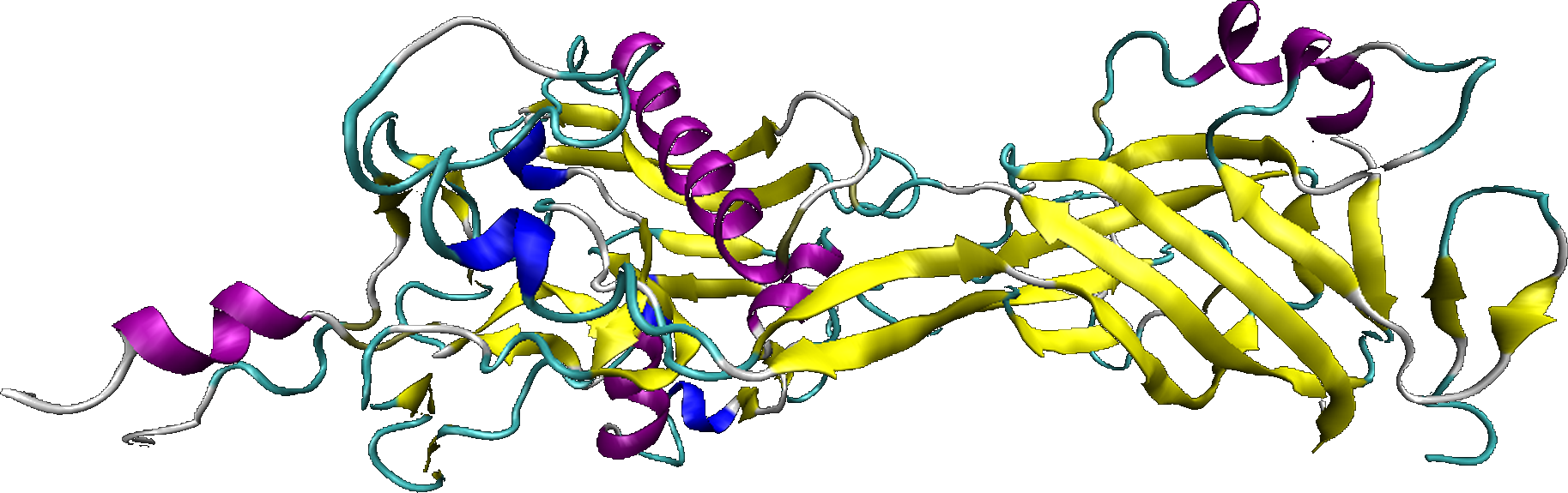
The internalization protein, called polypeptide III, is issued of the adenovirus penton base. This one allows to the cell vector to be internalized in the interest cell, and to induce the endosomal escape.
The penton base is a pentameric protein strategically inserted in the twelve peaks of the adenoviral capsid. Its monomer, which the length is around 60 kDa (571 aminoacides), is present in 60 copies in the viruses. More than its structural function in the capsid, the penton base has a fundamental property: it contains an RGD motif (arginin - aspartic acid - glycin) conserved, responsible of the interaction of the virus with the integrins αvβ3 and αvβ5. This motif is located at the penton base surface and is exposed to the outer of the capsid.
The exposition of 5 RGD motifs carries by the penton base, allows the simultaneous association of several integrin molecules, which initiate the incorporation of the adenovirus by endocytosis, principally mediated by clathrin vesicles.
The integrins gathering via the RGD motif is necessary for the internalization phenomenon. The gathering needs to several polypeptides III monomers. This can reveal a problem, indeed, only one monomer of polypeptide III is fused to one protein D, so the endocytosis could not happen.
However, since a crystallography study, the distance between two RGD motifs on one penton base is equal to 5,7nm. The distance between two proteins D is similar (5,1nm), so the integrins gathering can be achieved and induces the internalization and the endosomal escape.
Because of the fact that the lambda phage is naturally infectious for the prokaryotic organisms, the cellular internalization and the endosomal escape are serious limiting factors for its use to transfect eukaryotic cells. But, some studies have shown that the penton base improves in a significant way the cellular internalization and the endosomal escape.
Without any penton base, a phage looses almost the totality of its transfection efficiency. It is an important element for the maintain of a good transfection efficienty of the cell vector.
The adding of the internalization protein, the polypeptide III, allows to the cell vector, the internalization and the endosomal escape of the eukaryotic cells. Once outside of the endosome, the therapeutic plasmid is released in the cytoplasm of the targeted cell and can act.
To summarize…
The DVS has a cell vector , with the following characteristics:
- Non pathogenic so not toxic,
- Cell targeting, so the 2nd specificity,
- Passage of the eukaryotic cells membrane,
- Encapsidation and delivery of a specific plasmid.
This characteristics, specific to the DVS, bring a solution to the redundant problems, of specificity, membrane passage, and therapeutic agent delivery, encounter by the vectors.
 "
"