Team:SupBiotech-Paris/Safety
From 2009.igem.org
(→BioBricks) |
Enguerrand (Talk | contribs) (→Laboratory safety) |
||
| (14 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:Supbiotechcss9.css}} | {{Template:Supbiotechcss9.css}} | ||
{{Template:SupbiotechparisEn}} | {{Template:SupbiotechparisEn}} | ||
| - | + | http://img218.imageshack.us/img218/3930/safety.png | |
| - | + | ||
== Judging form == | == Judging form == | ||
| - | '''Would any of your project ideas raise safety issues in terms | + | '''Would any of your project ideas raise safety issues in terms of''': <br> |
Researcher safety? | Researcher safety? | ||
| - | :*No, because all hygiene and security rules were followed. ([[Team:SupBiotech-Paris/Safety# | + | :*No, because all hygiene and security rules were followed. ([[Team:SupBiotech-Paris/Safety#Laboratory_safety|More...]]) <br> |
Public safety? | Public safety? | ||
| - | :*No, the DVS is not dangerous for humans. ([[Team:SupBiotech-Paris/Safety# | + | :*No, the DVS is not dangerous for humans. ([[Team:SupBiotech-Paris/Safety#Safety_for_society_and_the_environment|More...]])<br> |
Environmental safety? | Environmental safety? | ||
| - | :*No, it cannot be spread in the environment ([[Team:SupBiotech-Paris/Safety# | + | :*No, it cannot be spread in the environment ([[Team:SupBiotech-Paris/Safety#Safety_for_society_and_the_environment|More...]])<br> |
'''Is there a local biosafety group, committee, or review board at your institution?''' <br> | '''Is there a local biosafety group, committee, or review board at your institution?''' <br> | ||
| - | :*We do not have a department's safety in our school. However there is a committee that is responsible for validating any new project in the laboratory that welcomed us. ([[Team:SupBiotech-Paris/Safety# | + | :*We do not have a department's safety in our school. However there is a committee that is responsible for validating any new project in the laboratory that welcomed us. ([[Team:SupBiotech-Paris/Safety#Laboratory_safety|More...]])<br> |
''' What does your local biosafety group think about your project ?''' <br> | ''' What does your local biosafety group think about your project ?''' <br> | ||
| - | :*The head of the laboratory agreed to let us develop our project in their premises. Moreover, one of the managers attended our ethical debate ([[Team:SupBiotech-Paris/Safety# | + | :*The head of the laboratory agreed to let us develop our project in their premises. Moreover, one of the managers attended our ethical debate ([[Team:SupBiotech-Paris/Safety#Laboratory_safety|More...]])<br> |
''' Do any of the new BioBrick parts that you made this year raise any safety issues ?''' <br> | ''' Do any of the new BioBrick parts that you made this year raise any safety issues ?''' <br> | ||
| - | :*No, the parts are mostly associations of existing BioBricks. The other Biobrick parts do not involve new safety issues. ([[Team:SupBiotech-Paris/Safety#BioBricks| | + | :*No, the parts are mostly associations of existing BioBricks. The other Biobrick parts do not involve new safety issues. ([[Team:SupBiotech-Paris/Safety#BioBricks|More...]] )<br> |
| Line 111: | Line 110: | ||
==Safety in DVS project== | ==Safety in DVS project== | ||
| - | |||
| - | |||
| - | |||
| - | |||
===Safety for society and the environment=== | ===Safety for society and the environment=== | ||
| Line 138: | Line 133: | ||
Our cell vector encapsidated only the therapeutic plasmid (not the entire genome). If it infects bacteria of the commensal flora of the organism (which may be limited by changes in protein internalization), the bacteria will receive just the therapeutic plasmid. <br> | Our cell vector encapsidated only the therapeutic plasmid (not the entire genome). If it infects bacteria of the commensal flora of the organism (which may be limited by changes in protein internalization), the bacteria will receive just the therapeutic plasmid. <br> | ||
| - | Moreover, our phage is a prokaryote, but cells of human body are eukaryotes. It can therefore be no risk of homologous recombination or integration between its genome and our cells genome, as they do not belong to the same "world". <br> | + | Moreover, our phage is a prokaryote, but cells of human body are eukaryotes. It can therefore be no risk of homologous recombination or integration between its genome and our cells genome, as they do not belong to the same "world".<br> |
| Line 154: | Line 149: | ||
| - | + | Our biobricks set no safety problems : | |
| - | + | *The biobrick for COS sequence may set a security problem since it allows the encapsidation of any sequence within the bacteriophage lambda. We can therefore encapsidate “harmful” sequences in a recombinant phage. But this biobrick was not subjected to iGEM. <br> | |
| - | + | *Some of our biobricks are an assembling of bricks already existing in the register of MIT. <br> | |
| - | + | *For other biobricks, it is a DNA targeting sequence (DTS), which recruits nuclear localization sequence, a factor specific stem cells (CFS) and a capsid protein of phage lambda synthesized from its own genome. Thus it does not cover new issues of safety. <br> | |
| - | + | ||
| Line 171: | Line 165: | ||
Good laboratory practices for the experiments on mice were also followed. (Renewal of litter, respect of the animal, reducing the number of animals used...) <br> | Good laboratory practices for the experiments on mice were also followed. (Renewal of litter, respect of the animal, reducing the number of animals used...) <br> | ||
| - | |||
| Line 180: | Line 173: | ||
<div style="float: right; margin-right: -85px;"> | <div style="float: right; margin-right: -85px;"> | ||
<a href="https://2009.igem.org/Team:SupBiotech-Paris/Gallery#drapeau" target="_self"> | <a href="https://2009.igem.org/Team:SupBiotech-Paris/Gallery#drapeau" target="_self"> | ||
| - | <img title="Let's go to the next page !" style="width: 100px;" src="https://static.igem.org/mediawiki/2009/ | + | <img title="Let's go to the next page !" style="width: 100px;" src="https://static.igem.org/mediawiki/2009/b/b5/Bibsette2.png";> |
</a></div> | </a></div> | ||
</html> | </html> | ||
Latest revision as of 02:26, 22 October 2009
http://img218.imageshack.us/img218/3930/safety.png
Contents |
Judging form
Would any of your project ideas raise safety issues in terms of:
Researcher safety?
- No, because all hygiene and security rules were followed. (More...)
- No, because all hygiene and security rules were followed. (More...)
Public safety?
- No, the DVS is not dangerous for humans. (More...)
- No, the DVS is not dangerous for humans. (More...)
Environmental safety?
- No, it cannot be spread in the environment (More...)
- No, it cannot be spread in the environment (More...)
Is there a local biosafety group, committee, or review board at your institution?
- We do not have a department's safety in our school. However there is a committee that is responsible for validating any new project in the laboratory that welcomed us. (More...)
- We do not have a department's safety in our school. However there is a committee that is responsible for validating any new project in the laboratory that welcomed us. (More...)
What does your local biosafety group think about your project ?
- The head of the laboratory agreed to let us develop our project in their premises. Moreover, one of the managers attended our ethical debate (More...)
- The head of the laboratory agreed to let us develop our project in their premises. Moreover, one of the managers attended our ethical debate (More...)
Do any of the new BioBrick parts that you made this year raise any safety issues ?
- No, the parts are mostly associations of existing BioBricks. The other Biobrick parts do not involve new safety issues. (More... )
- No, the parts are mostly associations of existing BioBricks. The other Biobrick parts do not involve new safety issues. (More... )
Safety for Synthetic Biology
Foreword
Synthetic biology is a new approach of biology, which aims to synthesize and design new biological components and systems or redesign existing biological elements in order to create systems performing a specific function.
Synthetic biology has grown very rapidly: it has enabled the development of new markets and the reorganization of different actors from biotechnology, energy, and pharmaceuticals to food processing and petrochemicals sectors. Currently, the activities of these companies are separated into two distinct groups: the one of Gene Foundries that synthesize genes and more complex systems on demand, and the one combining the companies developing microorganisms able of producing biofuels, drugs or chemicals.
These many applications show the interest of synthetic biology to industry and the various benefits it can bring to society in the future.
However, these advances should not be to the detriment of the safety and security for society. It is therefore necessary to study thoroughly the balance “benefit / risk “ before developing a new use of synthetic biology. Taking into account all these elements is necessary to develop solutions and performing protocols to reassure the society, and thus ensure the sustainability of this new approach of biology.
Introduction
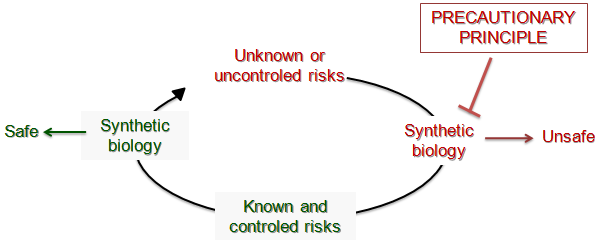
The main concern of the scientific community with regard to synthetic biology is that we cannot really predict the risks. Indeed, current knowledge does not allow us to have hindsight in order to predict the evolution and behavior of synthetic microorganisms. The engineering of biological systems is currently expensive and hazardous mainly because the scientific community does not properly control the molecular and cellular processes to provide reliable solutions.
Three different strategies have been identified to address this problem:
- The first is STANDARDIZATION. It consists in developing and promoting standards which we can also apply the definition, description and characterization of basic biological entities. The creation of the MIT Registry of Standard Biological Parts is a first step in this direction.
- The second is the DISSOCIATION. This method allows separating a complex problem into a number of simple problems more important. We can thus advance in solving various independent problems, and therefore solve complex systems.
- The last one is ABSTRACTION. It classifies information that describes the different biological functions, according to a hierarchy (which takes into account different levels of complexity).
These different strategies help to normalize and standardize the protocols and methods of synthetic biology in order to ease the use and thus limit the risks.
Pending greater use of these three strategies, it is necessary to anticipate the potential risks for experimentation that will be developed in the near future for the standardization of synthetic biology.
Synthetic biology is only in its infancy; it is difficult to determine potential risks. Nevertheless, to discern risk areas, we can transpose the experience gained during the development of recombinant DNA to synthetic biology.
We can then distinguish three major types of risks:
- The first one, microorganisms can escape from their confinement area, proliferate without any control and contaminate the environment, causing damage repairable or not.
- Secondly, synthetic organisms may develop in an open environment, side effects not detected during testing phases.
- Finally, it is also important to take into account the risk of bioterrorism. Terrorist organizations or individual actions can exploit synthetic biology for hostile purposes.
Accidental release
It has never yet been mentioned any release of GMOs into the environment. It is likely that GMO is less competitive compared to wild strains and did not persist in the environment. However, to avoid overflow, the scientific community has developed various means of containment:
- Physical containment: This is the most used; it ranks bacteria and pathogens agents into 4 classes and determines the requirements to be followed in handling.
- The trophic containment: It is about making the microorganism dependent of rare substances or unknown in nature, so it cannot grow without human intervention.
- The containment semantics: It is still in development. It consists for example on modifying the genetic code or the development of new nucleic acids, called XNA which the sugar is neither a desoxyribose or ribose, which prevents the transmission of genes.
A final way would be the addition of suicide genes into the genome of the microorganism, destroying the bacteria once performed its function.
Tests in open environment
Since the creation of GMOs and their use in agriculture, many varieties of plants were grown in nature: in an open environment, while the vast majority of species were only tested in the laboratory.
In theory, two types of adverse effects may occur after the test of a GMO in an open environment. A fortiori it is the case for Genetically Synthesized organisms (OGS):
- The microorganism can disturb the local biotope by creating a competitive environment, which may in the worst case lead to the extinction of several species.
- It may also, after having colonized the middle, becoming impossible to remove.
Bioterror
The rapid development of synthetic biology allows us to create living organisms de novo by enfranchising from the problems of genetic engineering. Many companies have developed to design new genes, segments of DNA and proteins. That popularity has increased access to technology for all. Indeed, sites for bio-yourselfers Sunday "flourish on the Internet, providing knowledge, tips and tricks and also professional equipment.
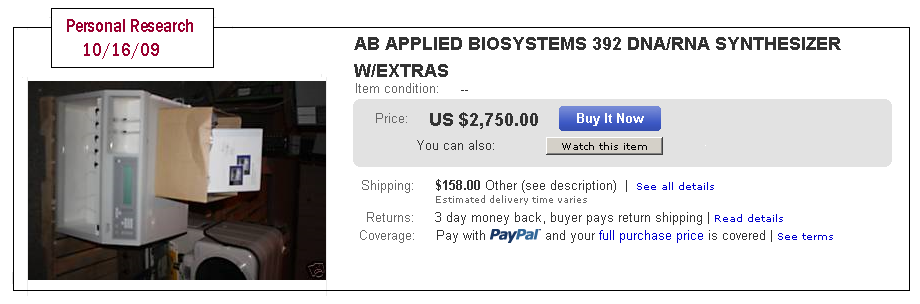 recherche ebay
recherche ebay
This personal research conducted on the site [www.ebay.com] demonstrated.
Although in most cases the bio-pirates have no intent to defraud, some persons pursue less noble goals:
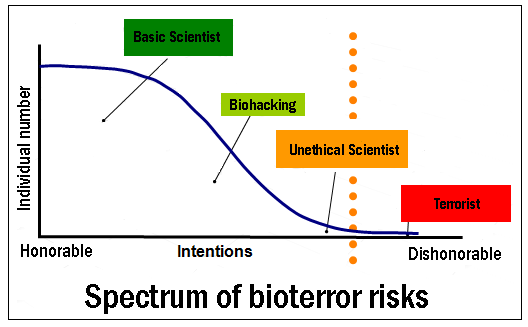
Many conferences on biosafety have highlighted the need to develop new techniques to reduce the risk of bio-terror.
- The first is simply to give a sense of responsibility to companies delivering genes by command. They have to verify the recipient of synthesized genes and also the type of genes required (compared with the genomes of known viruses and their toxins). However this cannot be set up in SMEs because it requires great financial resources that reduce the net profits of the company.
- The second is the research of defense strategies to fight against bio-terrorism.
- A final technique would also list all machines that can help to synthesize new genes, even if it is costly in time and financial resources.
Conclusion
In society today, many people are favorable to reduce and regulate the practice of synthetic biology until all risks are identified and excluded in accordance with the principle of precaution. However the application of this principle highlights a paradox, which harms innovation:
To use the techniques of synthetic biology, all risks must be known and controlled. But these risks cannot be determined if we do not progress by practicing synthetic biology. This is what the precautionary principle refuses because synthetic biology is not yet reliable.
The precautionary principle refuses innovation, as long as there is the slightest risk.
Conversely, the principle of responsibility can focus its work on the most serious risk after having considered all the risks. It is a conceivable solution to establish regulations; it allows pursuing researches despite uncontrolled risks.
Safety in DVS project
Safety for society and the environment
Mycobacterium avium subtype avium
Mycobacterium avium subtype avium is rarely responsible for serious infections in humans. As Willy Rozenbaum (co discoverer of HIV) said at our ethical debate, "is a bacterium that is very ubiquitous, it is found in faucet water, we are almost all contaminated.
Mycobacterium avium is "safe" for the environment and also for healthy people. Indeed, if there is an infection, it is localized in the organism. Conversely, infection is more global for immunodepressed patients (due to HIV for example). The effects of infection are, of course, to analyze in different phenotypes (healthy, tumor, immunodepressed) to investigate the benefit / risk balance for the patient.
Moreover, our Mycobacterium does not subsist in the organism. It is indeed lysed during the liberation of phage in the tissue after the addition of doxycycline.
Regarding doxycycline, it has side effects such as staining of bones, toxic epidermal necrolysis, the Stevens Johnson syndrome, megaloblastic anemia, esophageal ulceration, neuromuscular block, cutaneous porphyria, or dyschromatopsia. However, this antibiotic is already widely used by the pharmaceutical industry and does not present any danger as long as the doses and contraindication are respected.
Lambda Phage
Our cell vector encapsidated only the therapeutic plasmid (not the entire genome). If it infects bacteria of the commensal flora of the organism (which may be limited by changes in protein internalization), the bacteria will receive just the therapeutic plasmid.
Moreover, our phage is a prokaryote, but cells of human body are eukaryotes. It can therefore be no risk of homologous recombination or integration between its genome and our cells genome, as they do not belong to the same "world".
Therapeutic Plasmid
The therapeutic plasmid brings to the organism tumor suppressor genes (p53, p16, ATM, RB1 or PTEN) wild type. The promoters added are those present in healthy cells: so there is no increased synthesis of tumor suppressor genes in healthy cells. The regulation is not modified.
BioBricks
Our biobricks set no safety problems :
- The biobrick for COS sequence may set a security problem since it allows the encapsidation of any sequence within the bacteriophage lambda. We can therefore encapsidate “harmful” sequences in a recombinant phage. But this biobrick was not subjected to iGEM.
- Some of our biobricks are an assembling of bricks already existing in the register of MIT.
- For other biobricks, it is a DNA targeting sequence (DTS), which recruits nuclear localization sequence, a factor specific stem cells (CFS) and a capsid protein of phage lambda synthesized from its own genome. Thus it does not cover new issues of safety.
Laboratory safety
We do not have a department's safety in our school. However in the laboratory that welcomed us, there is a committee that is responsible for validating any new project.
During various experiments, a strict physical containment of our microorganisms was achieved and all the rules in the laboratory were followed. (Fume hood for handling dangerous substances, sterile containers, sorting waste, contaminated waste discharged into biological bins...)
Good laboratory practices for the experiments on mice were also followed. (Renewal of litter, respect of the animal, reducing the number of animals used...)
 "
"