Team:SupBiotech-Paris/Tissue targeting
From 2009.igem.org
(→Experimental protocol details) |
(→Experimental method) |
||
| (7 intermediate revisions not shown) | |||
| Line 10: | Line 10: | ||
== Objective == | == Objective == | ||
| - | We decided to check and quantify the | + | We decided to check and quantify the <i>Mycobacterium avium</i> presence, the tissue vector, inside lungs, during the intraveinous administration. Thus, we realized an <i>in vivo</i> study in several murine models.<br> |
| Line 17: | Line 17: | ||
== Experimental method == | == Experimental method == | ||
| - | To realize the <i>in vivo</i> study , we designed a bioluminescence protocol, which allows the real-time monitoring, of our tissue vector propagation in mice. The bioluminescence is a good reporter system to analyze the mycobacterial implantation and the clearance <i>in vivo</i>. We used a tool kindly provided by Dr Brian D. Robertson (Imperial College London researcher). This tool is a plasmid, once electroporated in M. <i>avium</i> can produce the firefly luciferase, a reporter gene, allowing the monitoring. Indeed, the luciferase catalysis the luciferin oxidation into oxiluciferin, involving photons emission, which can be caught by a CCD camera photosensitive (IVIS, Imaging System 50, Xenogen).<br> | + | To realize the <i>in vivo</i> study, we designed a bioluminescence protocol, which allows the real-time monitoring, of our tissue vector propagation in mice. The bioluminescence is a good reporter system to analyze the mycobacterial implantation and the clearance <i>in vivo</i>. We used a tool kindly provided by Dr Brian D. Robertson (Imperial College London researcher). This tool is a plasmid, once electroporated in M. <i>avium</i> can produce the firefly luciferase, a reporter gene, allowing the monitoring. Indeed, the luciferase catalysis the luciferin oxidation into oxiluciferin, involving photons emission, which can be caught by a CCD camera photosensitive (IVIS, Imaging System 50, Xenogen).<br> |
Unfortunately, the growth of electroporated mycobacteria with the monitoring plasmid, was so slow, it was not possible to realize this study within the given time (generation time of M. <i>avium</i>: nearly 20 hours. That is why we decided to change our protocol.<br> | Unfortunately, the growth of electroporated mycobacteria with the monitoring plasmid, was so slow, it was not possible to realize this study within the given time (generation time of M. <i>avium</i>: nearly 20 hours. That is why we decided to change our protocol.<br> | ||
Following this lack of time, we used another protocol (detailed in the following subpart) to check and quantify the presence of our tissue vector in the lung. For this, we studied in the literature what dose of M.<i>avium</i> could be injected into a mouse model. Different studies, on several pathologies of bacterial origin, mentioned the injection of 105 to 107 CFU.ml-1 (colony forming units) per mouse by intravenous route.<br> | Following this lack of time, we used another protocol (detailed in the following subpart) to check and quantify the presence of our tissue vector in the lung. For this, we studied in the literature what dose of M.<i>avium</i> could be injected into a mouse model. Different studies, on several pathologies of bacterial origin, mentioned the injection of 105 to 107 CFU.ml-1 (colony forming units) per mouse by intravenous route.<br> | ||
| Line 31: | Line 31: | ||
=== Experimental protocol details === | === Experimental protocol details === | ||
| - | :* Day -7: Inoculation of 10^ | + | :* Day -7: Inoculation of 4.10^6 LBS cells (fibroblastic cancer cells) subcutaneously on 2 black 6 mice.<br> |
:* Day 0: Inoculation of 10^6 CFU.ml-1* of tissue vectors, M.avium, by IV route (intravenous) injected in the tail of the 3 mouse models: immunodepressed, cancer, and normal (2 mice per model).<br> | :* Day 0: Inoculation of 10^6 CFU.ml-1* of tissue vectors, M.avium, by IV route (intravenous) injected in the tail of the 3 mouse models: immunodepressed, cancer, and normal (2 mice per model).<br> | ||
:: The concentration of injected tissue vectors was determined by measuring the optical density. The optical density of 10^6 bacteria is not detectable in a spectrophotometer. We therefore measured the cell suspension absorbance of 10^8 bacteria. Once diluted by 100, we obtained the desired concentration is: 10^6 bacteria.<br> | :: The concentration of injected tissue vectors was determined by measuring the optical density. The optical density of 10^6 bacteria is not detectable in a spectrophotometer. We therefore measured the cell suspension absorbance of 10^8 bacteria. Once diluted by 100, we obtained the desired concentration is: 10^6 bacteria.<br> | ||
| Line 44: | Line 44: | ||
== Results == | == Results == | ||
| - | A lung sample of each murine models, has been analysed by flow cytometry, to determine and quantify our tissue vector presence, M.<i>avium</i> in the lungs. This experiment could not be repeated more than once, | + | A lung sample of each murine models, has been analysed by flow cytometry, to determine and quantify our tissue vector presence, M.<i>avium</i> in the lungs. This experiment could not be repeated more than once, we can only be reserved for these results. Nevertheless, various publications have already demonstrated the <i>Mycobacterium avium</i> implantation in the lungs, thus proving the credibility and feasibility of this study.<br> |
[[Image : Temoincyto.png|center|600px]] | [[Image : Temoincyto.png|center|600px]] | ||
| - | + | From the results above, we determined the signature of a sample of <i>Mycobacterium avium </i> by flow cytometry (red zone R1). The left image corresponds to background noise or signal of the cytometer. However, It would have been very interesting to make a 3rd control, with the acquisition of a sample of lungs of mice not infected with <i> M.avium </i>.<br> | |
[[Image : Echancyto1En.png|center|600px]] | [[Image : Echancyto1En.png|center|600px]] | ||
| Line 67: | Line 67: | ||
[[Image : Graphpoumon.png|center|200px|float]] | [[Image : Graphpoumon.png|center|200px|float]] | ||
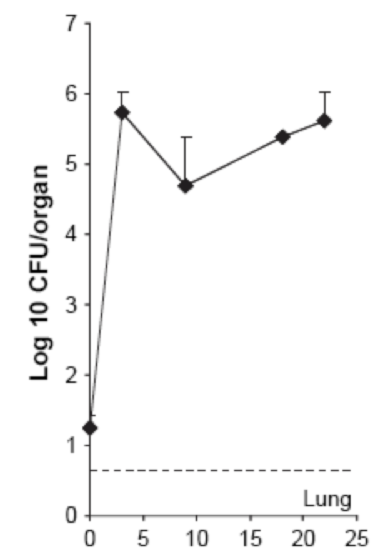
| - | The above figure is extracted from the publication « [[Team:SupBiotech-Paris/ | + | The above figure is extracted from the publication « [[Team:SupBiotech-Paris/Bibliography#revulung|Thymus as a target for mycobacterial infections]] ». A similar protocol was performed with injection of 10 ^ 6 CFU / ml <i> M. <i> avium </i> administered intravenously. . It is described in this figure that the lungs are infected from the first day of infection, but in very small amount (just over 10 ^ 1, then it is more prolonged with the rapid spread (10 ^ 1 to 10 ^ 6 CFU / organ in less than 5 weeks). This proves, therefore, that the lungs are colonized by M.<i> avium </i>.<br> |
| Line 74: | Line 74: | ||
== Conclusion == | == Conclusion == | ||
| - | Although our | + | Although our experiment does not prove significantly the presence of <i>M. avium</i> in the lung, it has been proven many times that this type of bacteria colonizes the lung. This organ is a one of the natural tropisms of ''M. avium'' in mice and humans.<br> |
| - | We can use the [[Team:SupBiotech-Paris/ | + | We can use the [[Team:SupBiotech-Paris/Concept#DVS|DVS]] on lung cancer, because we are confident that the [[Team:SupBiotech-Paris/Concept1#drapeau|vector tissue]] can target the organism and release the [[Team:SupBiotech-Paris/Concept2#drapeau|cell vector]].<br> |
Latest revision as of 01:32, 22 October 2009
Contents |
Tissue targeting
Context
The non-small cell lung cancer, or NSCLC, is a cancer, which develops in the organ lumen. This tumor localization is essentially due to factors triggering the tumorogenesis (example: tobacco). A logic route of administration for the treatment would be the aerosol route, though a nebulization of the bacteria. But, this route is not the one that we chose for our treatment. The DVS treatment applied to the lungs cancer is administrated by intravenous.
Objective
We decided to check and quantify the Mycobacterium avium presence, the tissue vector, inside lungs, during the intraveinous administration. Thus, we realized an in vivo study in several murine models.
Experimental method
To realize the in vivo study, we designed a bioluminescence protocol, which allows the real-time monitoring, of our tissue vector propagation in mice. The bioluminescence is a good reporter system to analyze the mycobacterial implantation and the clearance in vivo. We used a tool kindly provided by Dr Brian D. Robertson (Imperial College London researcher). This tool is a plasmid, once electroporated in M. avium can produce the firefly luciferase, a reporter gene, allowing the monitoring. Indeed, the luciferase catalysis the luciferin oxidation into oxiluciferin, involving photons emission, which can be caught by a CCD camera photosensitive (IVIS, Imaging System 50, Xenogen).
Unfortunately, the growth of electroporated mycobacteria with the monitoring plasmid, was so slow, it was not possible to realize this study within the given time (generation time of M. avium: nearly 20 hours. That is why we decided to change our protocol.
Following this lack of time, we used another protocol (detailed in the following subpart) to check and quantify the presence of our tissue vector in the lung. For this, we studied in the literature what dose of M.avium could be injected into a mouse model. Different studies, on several pathologies of bacterial origin, mentioned the injection of 105 to 107 CFU.ml-1 (colony forming units) per mouse by intravenous route.
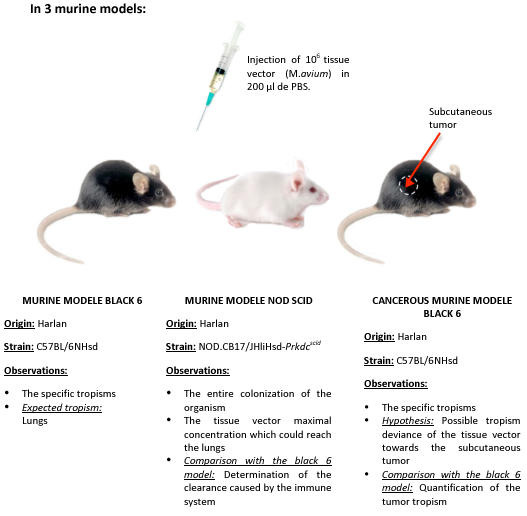
We chose to realize this experiment on 3 murine model: immunodepressed, normal, and cancerous.
According to several studies, it seems that mycobacteria has a specific affinity for fibronectins, surexpressed at the surface of tumor cells. Mycobacterial tropism for tumor area has not been yet demonstrated, the cancerous murine model allows us to test this hypothesis.
Experimental protocol details
- Day -7: Inoculation of 4.10^6 LBS cells (fibroblastic cancer cells) subcutaneously on 2 black 6 mice.
- Day 0: Inoculation of 10^6 CFU.ml-1* of tissue vectors, M.avium, by IV route (intravenous) injected in the tail of the 3 mouse models: immunodepressed, cancer, and normal (2 mice per model).
- The concentration of injected tissue vectors was determined by measuring the optical density. The optical density of 10^6 bacteria is not detectable in a spectrophotometer. We therefore measured the cell suspension absorbance of 10^8 bacteria. Once diluted by 100, we obtained the desired concentration is: 10^6 bacteria.
- Day 7: Mice sacrifice, organs extraction and cell suspension obtention, from the lungs, liver, spleen.
- Day -7: Inoculation of 4.10^6 LBS cells (fibroblastic cancer cells) subcutaneously on 2 black 6 mice.
The cell suspension obtained is then analyzed by flow cytometry, to verify the size and granularity of cells. These 2 characteristics are totally different between eukaryotic cells comprising murine organs, and the vector tissue M.avium of prokaryotic origin.
We can thus verify and quantify the presence of the vector tissue.
Results
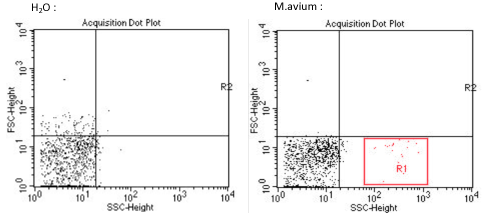
A lung sample of each murine models, has been analysed by flow cytometry, to determine and quantify our tissue vector presence, M.avium in the lungs. This experiment could not be repeated more than once, we can only be reserved for these results. Nevertheless, various publications have already demonstrated the Mycobacterium avium implantation in the lungs, thus proving the credibility and feasibility of this study.
From the results above, we determined the signature of a sample of Mycobacterium avium by flow cytometry (red zone R1). The left image corresponds to background noise or signal of the cytometer. However, It would have been very interesting to make a 3rd control, with the acquisition of a sample of lungs of mice not infected with M.avium .
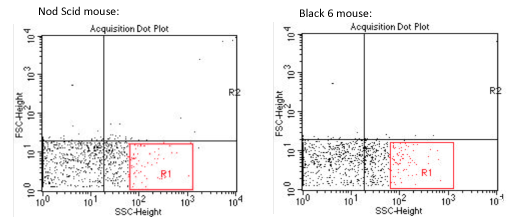
In the above samples, it is difficult to find specifically the signature of the « mycobacterium » sample. It is also very difficult to discern any difference between the 2 mouse model, Nod Scid and Black 6.
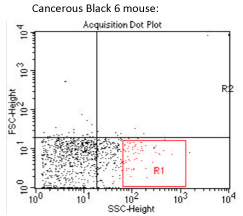
The opposite samples, does not allow us to determine a possible influence of the subcutaneous tumor on the mycobacterial tropism, or even to differentiate the M. avium tropism in this model, from others models.
Discussion
This experiment achieved only a single time, failed to meet our goals, to identify and quantify the M. avium tropisms. However, lack of time is another factor to consider. Indeed, this analysis by flow cytometry was performed one week after the injection of M. avium in mice. After reading various publications, it is interesting to note that the significant data of M. avium implantation in the lungs, are obtained after many weeks. Therefore, it explains our difficulty to quantify by flow cytometry, the presence of the vector tissue after one week.
The above figure is extracted from the publication « Thymus as a target for mycobacterial infections ». A similar protocol was performed with injection of 10 ^ 6 CFU / ml M. <i> avium administered intravenously. . It is described in this figure that the lungs are infected from the first day of infection, but in very small amount (just over 10 ^ 1, then it is more prolonged with the rapid spread (10 ^ 1 to 10 ^ 6 CFU / organ in less than 5 weeks). This proves, therefore, that the lungs are colonized by M. avium .
Conclusion
Although our experiment does not prove significantly the presence of M. avium in the lung, it has been proven many times that this type of bacteria colonizes the lung. This organ is a one of the natural tropisms of M. avium in mice and humans.
We can use the DVS on lung cancer, because we are confident that the vector tissue can target the organism and release the cell vector.
 "
"