Team:SupBiotech-Paris/Tissue targeting
From 2009.igem.org
Enguerrand (Talk | contribs) |
Enguerrand (Talk | contribs) |
||
| Line 2: | Line 2: | ||
{{Template:SupbiotechparisEn}} | {{Template:SupbiotechparisEn}} | ||
| + | = Tissue targeting = | ||
| - | <span style="float: right">[[Team:SupBiotech-Paris/ | + | == Context == |
| + | |||
| + | The non-small cell lung cancer, or NSCLC, is a cancer, which develops in the organ lumen. This tumor localization is essentially due to factors triggering the tumorogenesis (example: tobacco). A logic route of administration for the treatment would be the aerosol route, though a nebulization of the bacteria. But, this route is not the one that we chose for our treatment. The DVS treatment applied to the lungs cancer is administrated by intravenous.<br> | ||
| + | |||
| + | == Objective == | ||
| + | |||
| + | We decided to check and quantify the mycobacterium avium presence, the tissue vector, in side the lungs, during the intraveinous administration. Thus, we realized an <i>in vivo</i> study in several murine models.<br> | ||
| + | |||
| + | |||
| + | <span style="float: right">[[Team:SupBiotech-Paris/Tissue_targeting#drapeau|Back to top]]</span> | ||
| + | |||
| + | == Experimental method == | ||
| + | |||
| + | To realize the <i>in vivo</i> study , we designed a bioluminescence protocol, which allows the real-time monitoring, of our tissue vector propagation in mice. The bioluminescence is a good reporter system to analyze the mycobacterial implantation and the clearance <i>in vivo</i>. We used a tool kindly provided by Dr Brian D. Robertson (Imperial College London researcher). This tool is a plasmid, once electroporated in M. <i>avium</i> can produce the firefly luciferase, a reporter gene, allowing the monitoring. Indeed, the luciferase catalysis the luciferin oxidation into oxiluciferin, involving photons emission, which can be caught by a CCD camera photosensitive (IVIS, Imaging System 50, Xenogen).<br> | ||
| + | Unfortunately, the growth of electroporated mycobacteria with the monitoring plasmid, was so slow, it was not possible to realize this study within the given time (generation time of M. <i>avium</i>: nearly 20 hours. That is why we decided to change our protocol.<br> | ||
| + | Following this lack of time, we used another protocol (detailed in the following subpart) to check and quantify the presence of our tissue vector in the lung. For this, we studied in the literature what dose of M.<i>avium</i> could be injected into a mouse model. Different studies, on several pathologies of bacterial origin, mentioned the injection of 105 to 107 CFU.ml-1 (colony forming units) per mouse by intravenous route.<br> | ||
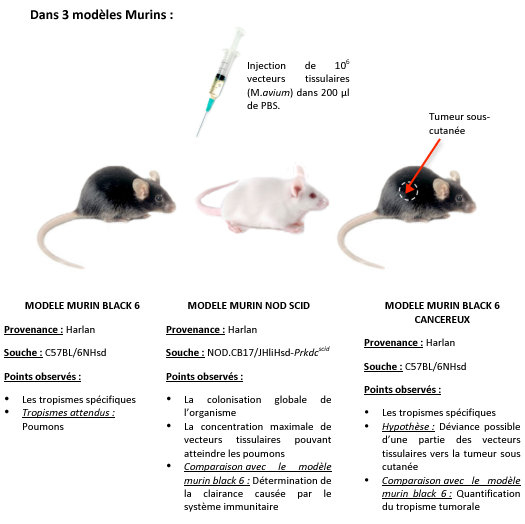
| + | We chose to realize this experiment on 3 murine model: immunodepressed, normal, and cancerous.<br> | ||
| + | |||
| + | [[Image : 3modelmurin.png|center|650px]] | ||
| + | |||
| + | According to several studies, it seems that mycobacteria has a specific affinity for fibronectins, surexpressed at the surface of tumor cells. Mycobacterial tropism for tumor area has not been yet demonstrated, the cancerous murine model allows us to test this hypothesis.<br> | ||
| + | |||
| + | |||
| + | <span style="float: right">[[Team:SupBiotech-Paris/Tissue_targeting#drapeau|Back to top]]</span> | ||
| + | |||
| + | === Experimental protocol details === | ||
| + | |||
| + | :* Day -7: Inoculation of 104 LBS cells (fibroblastic cancer cells) subcutaneously on 2 black 6 mice.<br> | ||
| + | :* Day 0: Inoculation of 106 CFU.ml-1* of tissue vectors, M.avium, by IV route (intravenous) injected in the tail of the 3 mouse models: immunodepressed, cancer, and normal (2 mice per model).<br> | ||
| + | :: The concentration of injected tissue vectors was determined by measuring the optical density. The optical density of 106 bacteria is not detectable in a spectrophotometer. We therefore measured the cell suspension absorbance of 108 bacteria. Once diluted by 100, we obtained the desired concentration is: 106 bacteria.<br> | ||
| + | :* Day 7: Mice sacrifice, organs extraction and cell suspension obtention, from the lungs, liver, spleen.<br> | ||
| + | |||
| + | The cell suspension obtained is then analyzed by flow cytometry, to verify the size and granularity of cells. These 2 characteristics are totally different between eukaryotic cells comprising murine organs, and the vector tissue M.avium of prokaryotic origin.<br> | ||
| + | We can thus verify and quantify the presence of the vector tissue.<br> | ||
| + | |||
| + | |||
| + | <span style="float: right">[[Team:SupBiotech-Paris/Tissue_targeting#drapeau|Back to top]]</span> | ||
| + | |||
| + | == Résultats == | ||
| + | |||
| + | A lung sample of each murine models, has been analysed by flow cytometry, to determine and quantify our tissue vector presence, M.<i>avium</i> in the lungs. This experiment could not be repeated more than once, we can we can only be reserved against thèse results. Nevertheless, various publications have already demonstrated the <i>Mycobacterium avium</i> implantation in the lungs, thus proving the credibility and feasibility of this study.<br> | ||
| + | |||
| + | [[Image : Temoincyto.png|center|600px]] | ||
| + | |||
| + | D’après les résultats ci-dessus, nous avons déterminé la signature d’un échantillon de <i>Mycobacterium avium</i> en cytométrie en flux (en rouge zone R1). L’image de gauche correspond au bruit de fond ou signaux parasites du cytomètre. Il aurait été cependant très intéressant de réaliser un 3e témoin, avec l’acquisition d’un échantillon de poumons de souris non infecté par <i>M. avium</i>.<br> | ||
| + | |||
| + | [[Image : Echancyto1.png|center|600px]] | ||
| + | |||
| + | In the above samples, it is difficult to find specifically the signature of the « mycobacterium » sample. It is also very difficult to discern any difference between the 2 mouse model, Nod Scid and Black 6. | ||
| + | |||
| + | [[Image : Echancyto12.png|center|300px|float|left]] | ||
| + | |||
| + | The opposite samples, does not allow us to determine a possible influence of the subcutaneous tumor on the mycobacterial tropism, or even to differentiate the <i>M. avium</i> tropism in this model, from others models.<br> | ||
| + | |||
| + | |||
| + | <span style="float: right">[[Team:SupBiotech-Paris/Tissue_targeting#drapeau|Back to top]]</span> | ||
| + | |||
| + | == Discussion == | ||
| + | |||
| + | This experiment achieved only a single time, failed to meet our goals, to identify and quantify the <i> M. avium </i> tropisms. However, lack of time is another factor to consider. Indeed, this analysis by flow cytometry was performed one week after the injection of <i> M. avium </i> in mice. After reading various publications, it is interesting to note that the significant data of <i>M. avium</i> implantation in the lungs, are obtained after many weeks. Therefore, it explains our difficulty to quantify by flow cytometry, the presence of the vector tissue after one week. <br> | ||
| + | |||
| + | [[Image : Graphpoumon.png|center|200px|float]] | ||
| + | |||
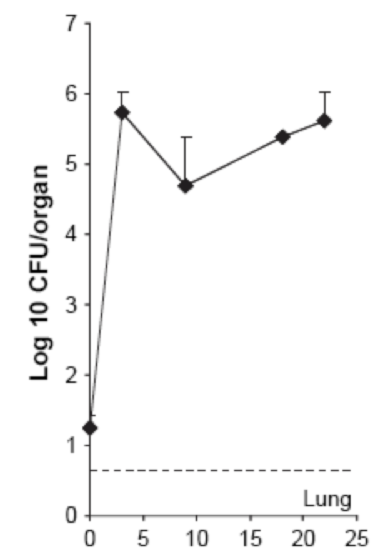
| + | La figure ci-dessus est tiré de la publication « [[Team:SupBiotech-Paris/Bibliographie#revulung|Thymus as a target for mycobacterial infections]] ». Le même type de protocole a été réalisé, avec injection de 10^6 CFU/ml de <i>M. avium</i> administré par voie intraveineuse. Il est décrit dans cette figure que les poumons sont infectés dès le premier jour de l’infection, mais en très petite quantité (légèrement supérieur à 10^1, puis de manière prolongée avec une propagation rapide (de 10^1 à 10^6 CFU/organe en moins de 5 semaines). Ceci prouve donc bien que les poumons sont colonisé par <i>M. avium</i>.<br> | ||
| + | |||
| + | |||
| + | <span style="float: right">[[Team:SupBiotech-Paris/Tissue_targeting#drapeau|Back to top]]</span> | ||
| + | |||
| + | == Conclusion == | ||
| + | |||
| + | Bien que notre étude ne prouve pas significativement la présence de <i>M. avium</i> dans le poumon, il a été prouvé mainte fois que ce type de bactérie colonise le poumon. Cet organe est l'un des tropismes naturelles de ''M. avium'' chez la souris, comme chez l’homme.<br> | ||
| + | On peut donc utiliser le [[Team:SupBiotech-Paris/Introduction1Fr#DVS|DVS]], sur le cancer du poumon, car nous avons la certitude que le [[Team:SupBiotech-Paris/Concept1Fr#drapeau|vecteur tissulaire]] peut cibler l'organe et y libérer le [[Team:SupBiotech-Paris/Concept2Fr#Drapeau|vecteur cellulaire]].<br> | ||
| + | |||
| + | |||
| + | <span style="float: right">[[Team:SupBiotech-Paris/Tissue_targeting#drapeau|Back to top]]</span> | ||
Revision as of 00:30, 21 October 2009
Contents |
Tissue targeting
Context
The non-small cell lung cancer, or NSCLC, is a cancer, which develops in the organ lumen. This tumor localization is essentially due to factors triggering the tumorogenesis (example: tobacco). A logic route of administration for the treatment would be the aerosol route, though a nebulization of the bacteria. But, this route is not the one that we chose for our treatment. The DVS treatment applied to the lungs cancer is administrated by intravenous.
Objective
We decided to check and quantify the mycobacterium avium presence, the tissue vector, in side the lungs, during the intraveinous administration. Thus, we realized an in vivo study in several murine models.
Experimental method
To realize the in vivo study , we designed a bioluminescence protocol, which allows the real-time monitoring, of our tissue vector propagation in mice. The bioluminescence is a good reporter system to analyze the mycobacterial implantation and the clearance in vivo. We used a tool kindly provided by Dr Brian D. Robertson (Imperial College London researcher). This tool is a plasmid, once electroporated in M. avium can produce the firefly luciferase, a reporter gene, allowing the monitoring. Indeed, the luciferase catalysis the luciferin oxidation into oxiluciferin, involving photons emission, which can be caught by a CCD camera photosensitive (IVIS, Imaging System 50, Xenogen).
Unfortunately, the growth of electroporated mycobacteria with the monitoring plasmid, was so slow, it was not possible to realize this study within the given time (generation time of M. avium: nearly 20 hours. That is why we decided to change our protocol.
Following this lack of time, we used another protocol (detailed in the following subpart) to check and quantify the presence of our tissue vector in the lung. For this, we studied in the literature what dose of M.avium could be injected into a mouse model. Different studies, on several pathologies of bacterial origin, mentioned the injection of 105 to 107 CFU.ml-1 (colony forming units) per mouse by intravenous route.
We chose to realize this experiment on 3 murine model: immunodepressed, normal, and cancerous.
According to several studies, it seems that mycobacteria has a specific affinity for fibronectins, surexpressed at the surface of tumor cells. Mycobacterial tropism for tumor area has not been yet demonstrated, the cancerous murine model allows us to test this hypothesis.
Experimental protocol details
- Day -7: Inoculation of 104 LBS cells (fibroblastic cancer cells) subcutaneously on 2 black 6 mice.
- Day 0: Inoculation of 106 CFU.ml-1* of tissue vectors, M.avium, by IV route (intravenous) injected in the tail of the 3 mouse models: immunodepressed, cancer, and normal (2 mice per model).
- The concentration of injected tissue vectors was determined by measuring the optical density. The optical density of 106 bacteria is not detectable in a spectrophotometer. We therefore measured the cell suspension absorbance of 108 bacteria. Once diluted by 100, we obtained the desired concentration is: 106 bacteria.
- Day 7: Mice sacrifice, organs extraction and cell suspension obtention, from the lungs, liver, spleen.
- Day -7: Inoculation of 104 LBS cells (fibroblastic cancer cells) subcutaneously on 2 black 6 mice.
The cell suspension obtained is then analyzed by flow cytometry, to verify the size and granularity of cells. These 2 characteristics are totally different between eukaryotic cells comprising murine organs, and the vector tissue M.avium of prokaryotic origin.
We can thus verify and quantify the presence of the vector tissue.
Résultats
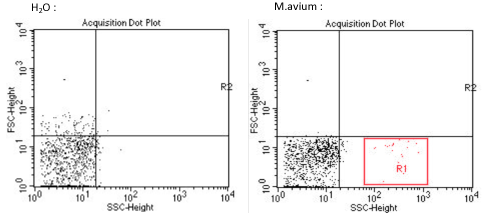
A lung sample of each murine models, has been analysed by flow cytometry, to determine and quantify our tissue vector presence, M.avium in the lungs. This experiment could not be repeated more than once, we can we can only be reserved against thèse results. Nevertheless, various publications have already demonstrated the Mycobacterium avium implantation in the lungs, thus proving the credibility and feasibility of this study.
D’après les résultats ci-dessus, nous avons déterminé la signature d’un échantillon de Mycobacterium avium en cytométrie en flux (en rouge zone R1). L’image de gauche correspond au bruit de fond ou signaux parasites du cytomètre. Il aurait été cependant très intéressant de réaliser un 3e témoin, avec l’acquisition d’un échantillon de poumons de souris non infecté par M. avium.
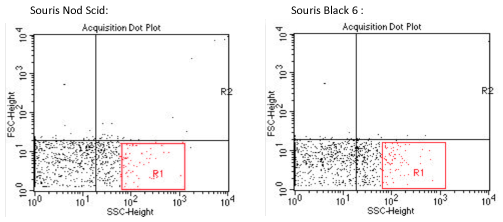
In the above samples, it is difficult to find specifically the signature of the « mycobacterium » sample. It is also very difficult to discern any difference between the 2 mouse model, Nod Scid and Black 6.
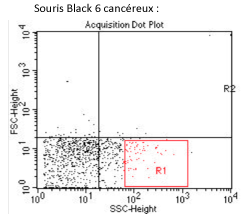
The opposite samples, does not allow us to determine a possible influence of the subcutaneous tumor on the mycobacterial tropism, or even to differentiate the M. avium tropism in this model, from others models.
Discussion
This experiment achieved only a single time, failed to meet our goals, to identify and quantify the M. avium tropisms. However, lack of time is another factor to consider. Indeed, this analysis by flow cytometry was performed one week after the injection of M. avium in mice. After reading various publications, it is interesting to note that the significant data of M. avium implantation in the lungs, are obtained after many weeks. Therefore, it explains our difficulty to quantify by flow cytometry, the presence of the vector tissue after one week.
La figure ci-dessus est tiré de la publication « Thymus as a target for mycobacterial infections ». Le même type de protocole a été réalisé, avec injection de 10^6 CFU/ml de M. avium administré par voie intraveineuse. Il est décrit dans cette figure que les poumons sont infectés dès le premier jour de l’infection, mais en très petite quantité (légèrement supérieur à 10^1, puis de manière prolongée avec une propagation rapide (de 10^1 à 10^6 CFU/organe en moins de 5 semaines). Ceci prouve donc bien que les poumons sont colonisé par M. avium.
Conclusion
Bien que notre étude ne prouve pas significativement la présence de M. avium dans le poumon, il a été prouvé mainte fois que ce type de bactérie colonise le poumon. Cet organe est l'un des tropismes naturelles de M. avium chez la souris, comme chez l’homme.
On peut donc utiliser le DVS, sur le cancer du poumon, car nous avons la certitude que le vecteur tissulaire peut cibler l'organe et y libérer le vecteur cellulaire.
 "
"