Team:TUDelft/31 July 2009
From 2009.igem.org
Lab Notebook
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
31 July 2009
Tim Weenink
Did some minipreps today. Then nanodropped them:
| Biobrick name | Internal reference | Concentration ng/µl | 260/280 | 260/230 |
| BBa_K142205 | *S2 | 86.4 | 2.01 | 2.03 |
| BBa_K142205 | *S3 | 107.7 | 2.03 | 2.11 |
| BBa_K142205 | *S4 | 69.0 | 1.97 | 1.96 |
| BBa_K142205 | *S5 | 87.4 | 1.98 | 1.99 |
| BBa_K142202 | *T1 | 128.5 | 1.99 | 2.09 |
| BBa_K142202 | *T2 | 129.4 | 2.02 | 2.16 |
| BBa_K142202 | *T3 | 126.6 | 1.97 | 2.06 |
| BBa_K142202 | *T4 | 106.7 | 1.95 | 2.05 |
| N/A | !A | 171.2 | 1.98 | 2.11 |
Sriram
Today I tested the competent cells prepared yesterday. For that I chose a three DNA samples. One is a diluted biobrick λp-rep for testing electro-competent cells, λp-R-GFP-T which had very high DNA concentration and biobrick pSB1K3 containing mRFP which had a normal DNA concentration for the two chemically competent cells prepared by TSS and TMF(RbCl) buffers respectively.
Orr helped me in doing the transformation of chemical competent cells prepared by TSS buffer. I did the transformation of those prepared by TMF buffer and electroporation. I have kept the plates in the draw to check the results on monday.
Calin
R751 cells arrived today. Cultures in all pSB4C5 tubes. Did miniprep on all tubes and also made a plate.
| Part | Concentration ng/µl | 260/280 | 260/230 |
| pSB4C5 I | 123.0 | 1.91 | 2.06 |
| pSB4C5 II | 125.8 | 1.92 | 2.29 |
| pSB4C5 III | 130.7 | 1.95 | 2.07 |
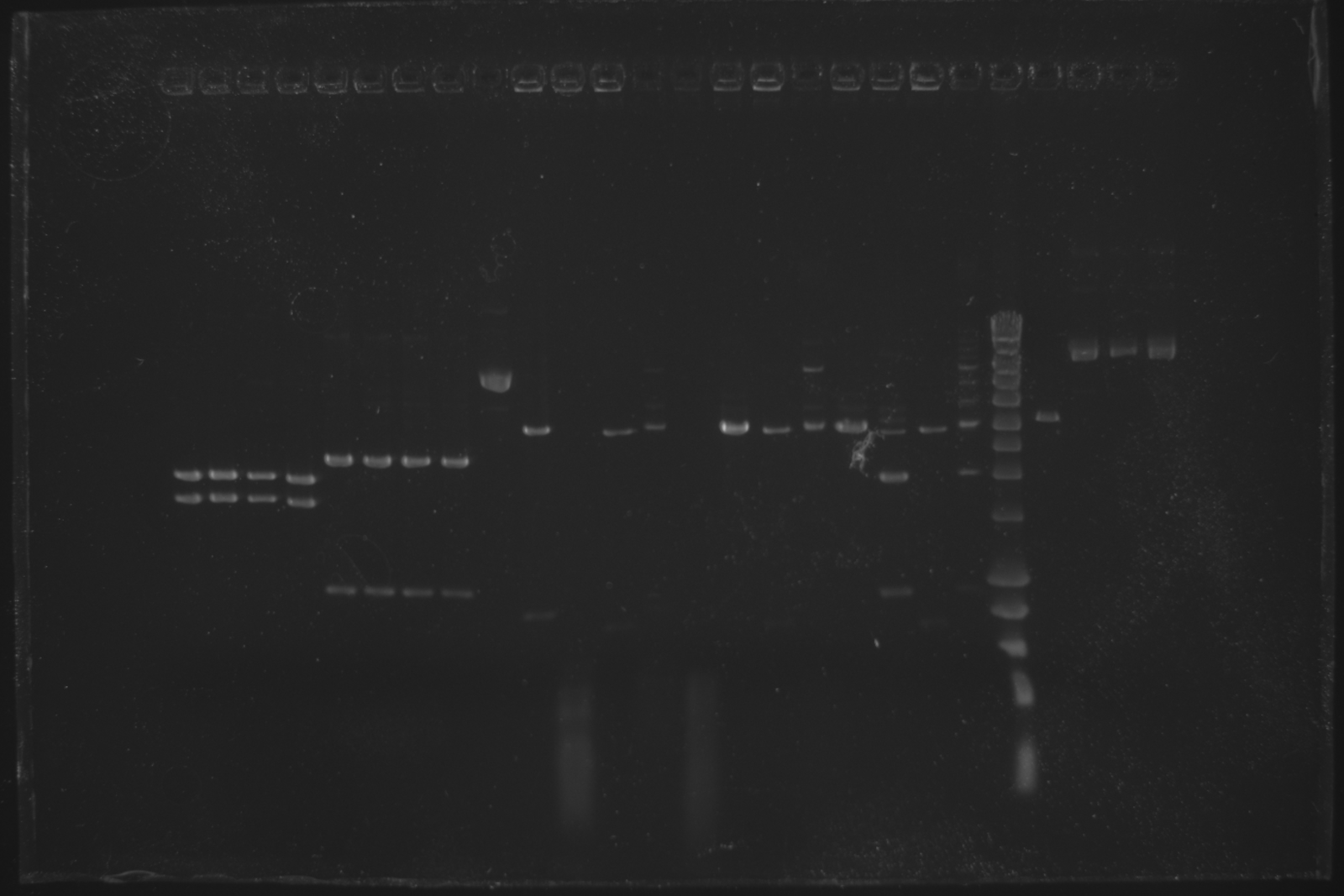
Todays gel confirmed that oriT-R digestion failed and pSB4C5 is way too large.
| Well | Part | Expected Plasmid Size | Status |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | |||
| 7 | |||
| 8 | |||
| 9 | |||
| 10 | |||
| 11 | |||
| 12 | |||
| 13 | |||
| 14 | |||
| 15 | |||
| 16 | |||
| 17 | |||
| 18 | |||
| 19 | |||
| 20 | |||
| 21 | |||
| 22 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✔ | |
| 23 | oriT E + P digest | 2079 | ✖ |
| 24 | pSB4C5 I | 3896 | ✖ |
| 25 | pSB4C5 II | 3896 | ✖ |
| 26 | pSB4C5 III | 3896 | ✖ |
Made two plates without antibiotics.
Digestions were started:
| position | parts | volume in µl |
| Upstream | BBa_I13522 pTet-GFP | 27.0 |
| H2O | 15.5 | |
| Upstream | BBa_E0840 rbs-GFP-term | 5.0 |
| H2O | 37.5 | |
| Backbone | pSB1C3 | 5.0 |
| H2O | 37.5 |
 "
"