Team:TUDelft/Modeling Cascade
From 2009.igem.org
Modeling the Transcriptional Cascade
The modeling of the transcriptional cascade had several objectives:
- to provide the delay team with design guidelines which would maximize the delay time
- to asses the affect of parameter variation on the delay time
- to determine areas of instability in the parameter space
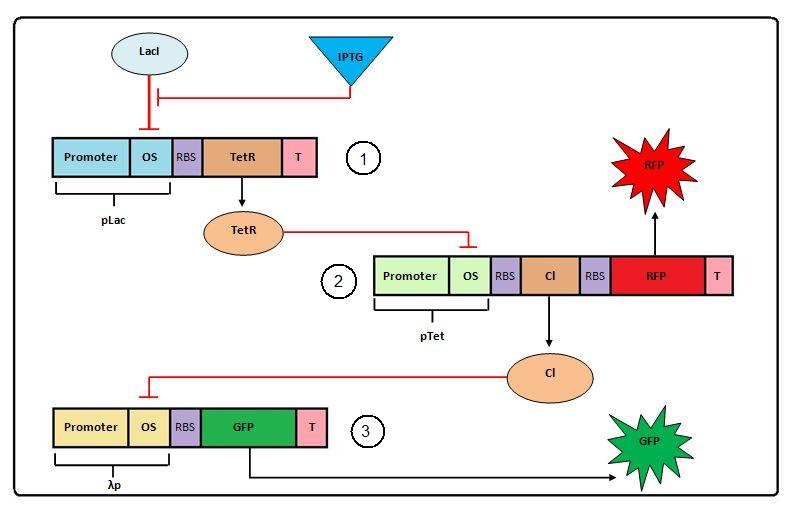
A schematic of the system to be modeled can be seen below. A full description of the Transcriptional Cascade can be found here.
The system shown above has one input: IPTG and one primary output: GFP. It is characterized by the delay time, which is measured as the time between the induction with IPTG and a significant expression in the final product (GFP).
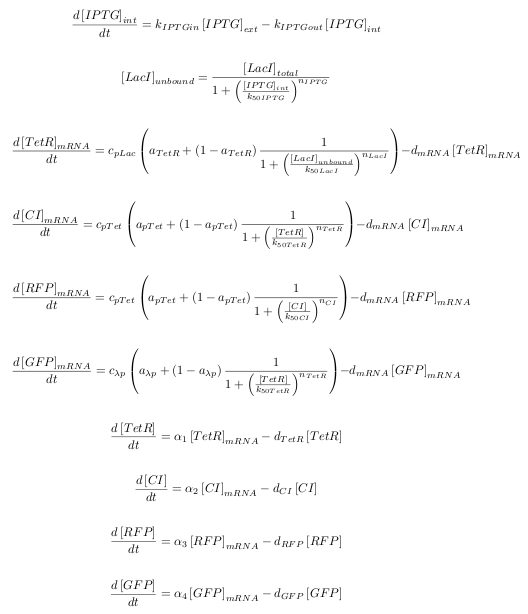
ODEs
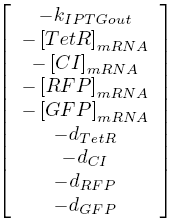
The kinetic equations were written out in a Matlab script. A total of ten equations were used: one for the diffusion of IPTG into the cell, one for the binding of IPTG to LacI, as well as four transcription equations, and four translation equations for the various levels of the cascade.
The notation in this system of equations can be seen in the table below:
| Symbol | Definition |
| kIPTGin, kIPTGout | rate constants |
| k50IPTG, k50LacI, k50TetR, k50CI | dissociation constants |
| dmRNA | mRNA degradation rate |
| dTetR, dCI, dRFP, dGFP | protein degradation rates |
| apLac, apTet, aλp | transcription leakage (%) |
| cpLac, cpTet, cλp | maximum transcription rates |
| α1, α2, α3, α4 | translation rates |
| nIPTG, nLacI, nTetR, nCI | Hill coefficients |
| [X]mRNA | concentration of X mRNA |
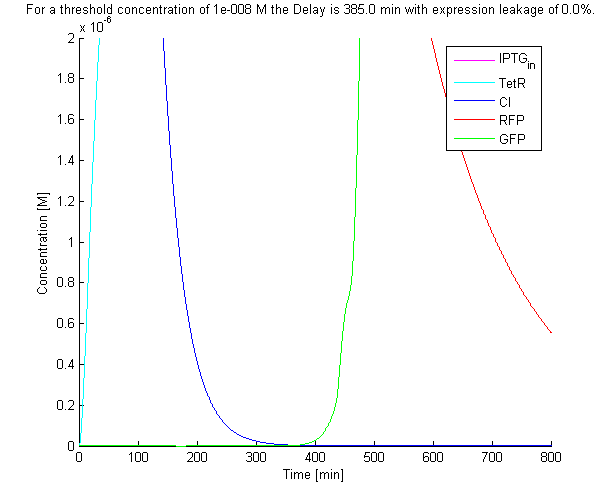
The solution with the default parameters of the system of ODEs can be seen below:
A function was written to determine the point at which the concentration of the final product reached a certain threshold. A threshold of 1E-8 M was used in our simulations, which corresponds to less than a dozen molecules within a cell given a cell volume of around 1E-15 L. Using the default parameter values a delay time of 385 min was predicted.
Sensitivity
A sensitivity analysis was done on the system. This looked at how variations in each parameter would influence the delay time. Parameters were swept over a range of values while the change in delay time was observed. The normalized sensitivity for various parameters is shown below.
| Parameter | Description | Normalized Sensitivity |
| apLac | transcription leakage of pLac | 0.00 |
| apTet | transcription leakage of pTet | 0.12 |
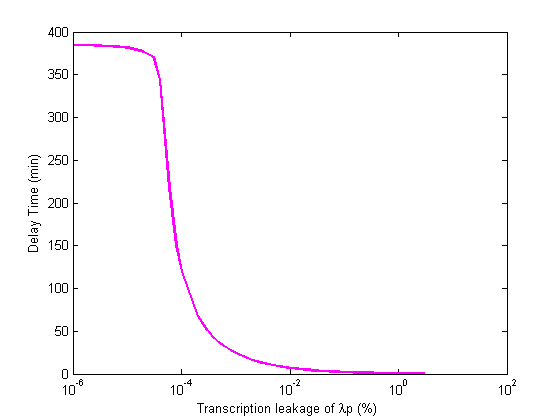
| aλp | transcription leakage of λp | 4.04 |
| cpLac | pLac promoter strength | 0.22 |
| cpTet | pTet promoter strength | 0.36 |
| cλp | λp promoter strength | 0.42 |
| α1 | TetR translation rate (RBS strength) | 0.14 |
| α2 | CI translation rate (RBS strength) | 1.06 |
As we can see in the table above, the system is most affected by changes in aλp, α2, followed by the strengths of the promoters used.
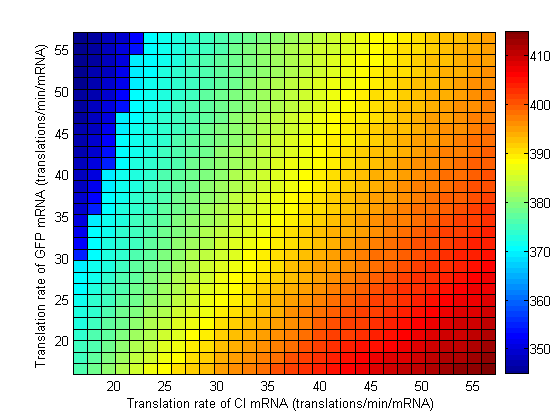
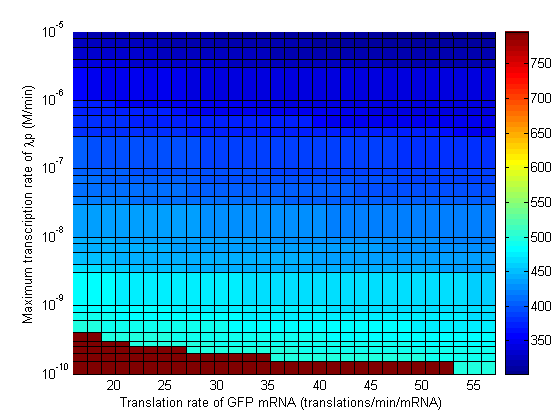
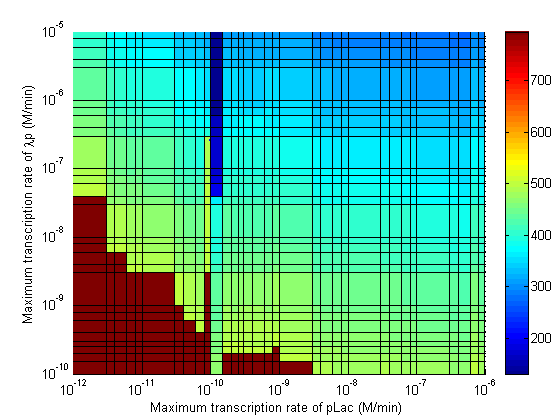
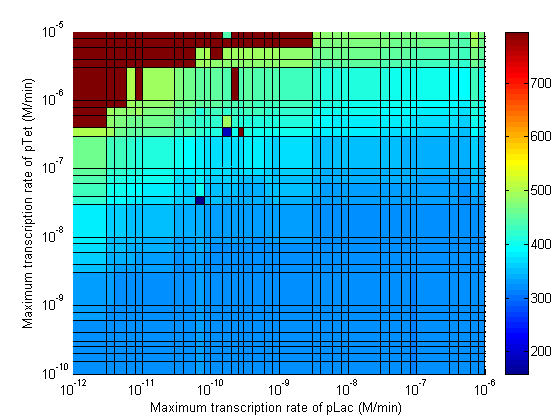
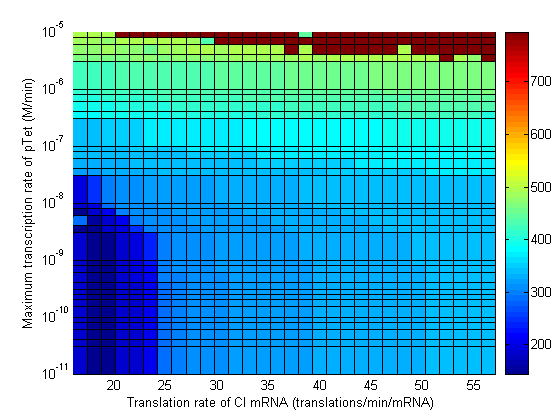
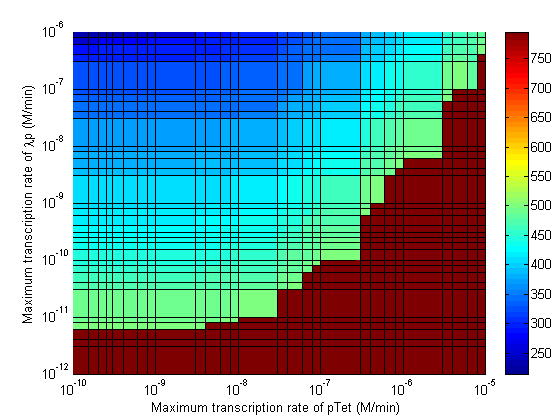
Parameter Sweeps
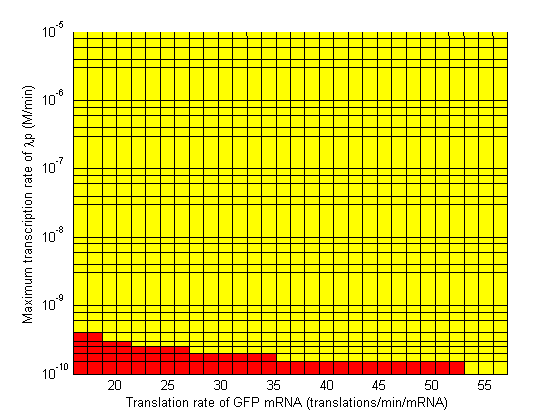
In the following plots the delay time of the cascade is shown as a function of two different parameters. A delay time off 500 is used to represent an infinite delay (maroon colour). The delay is considered to be over once the concentration of the final product (in this case GFP) goes above 10nM.
|
|
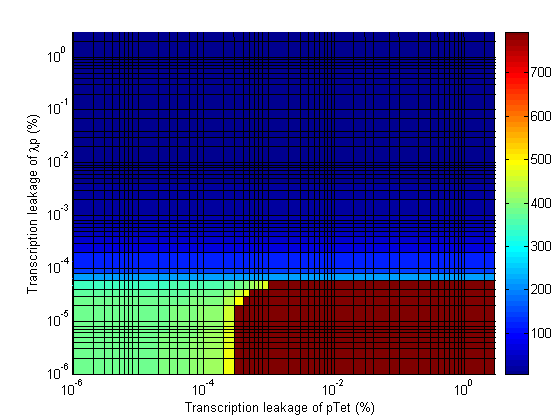
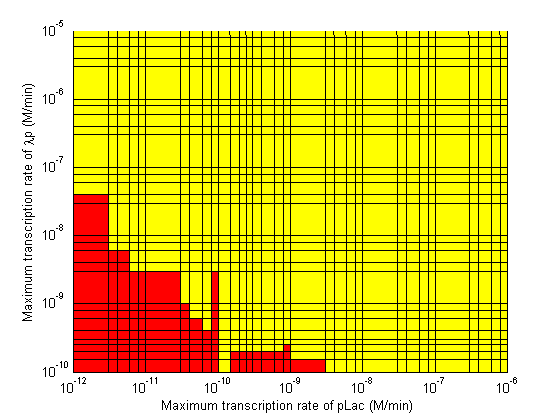
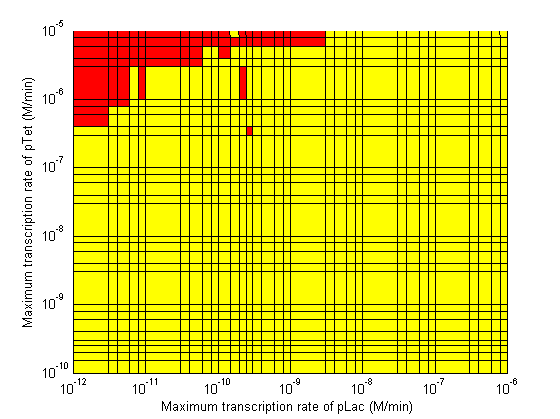
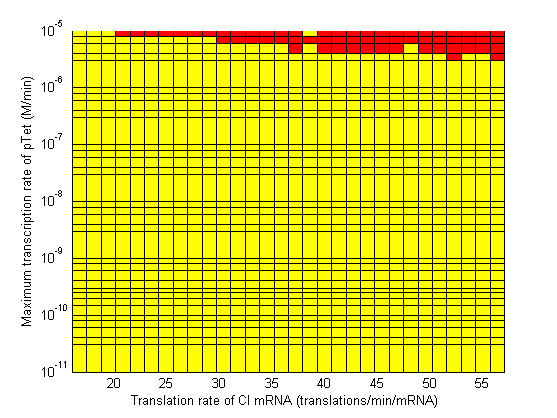
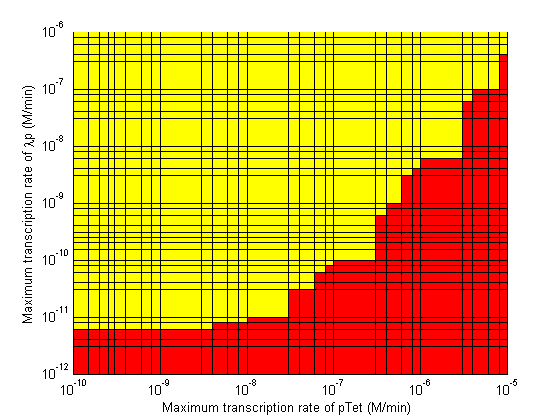
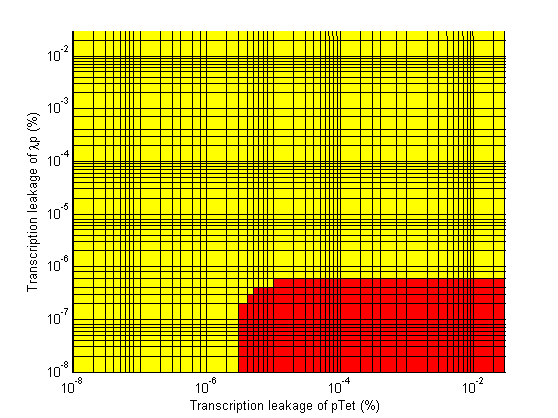
Stability
|
|
Design Recommendations
Based on the results of the simulations, a series of recommendations were given to the delay team to aid them in choosing parts which would maximize the delay time.
- Significant transcription leakages greatly shorten the delay time. Attempt to minimize leakages. Leakage of λp is a far bigger problem than pTet leakage.
- Use a weak promoter and a weak RBS on the last stage (λp) of the cascade.
- A weak pLac promoters is favorable.
- A strong pTet promoter is favorable.
- A strong RBS on CI gene is favorable.
- A weak RBS on TetR gene is favorable.
- A weak RBS on the endonuclease is favorable although a strong RBS can be used for the GFP gene.
- When choosing RBS and promoter strengths avoid the red areas on the stability plots.
 "
"