Team:USTC/Project
From 2009.igem.org
| Home | Team | Project | Modeling | Parts | Standard & Protocol | Software Tool | Human Practice | Notebook |
|---|
Team:USTC/Project
Contents
|
The Background
Evolution vs. Design
Evolution and design are two sides of the coin.
Until 150 years ago, people believed that we are designed and created by the God, a god, gods or other [http://en.wikipedia.org/wiki/Intelligent_designer intelligent designer]s. After [http://en.wikipedia.org/wiki/Charles_Darwin Charles Darwin]'s [http://en.wikipedia.org/wiki/On_the_Origin_of_Species On the Origin of Species] in 1859, more and more people began to realize that a god is not necessary. During the process of evolution, we can be created automatically, without any intelligence.
[http://en.wikipedia.org/wiki/Design Design] and [http://en.wikipedia.org/wiki/Engineering engineering] are processes with the need of knowledge. Before we can design a machine, we have to know everything about the possible parts. If any information is unknown, we have to learn it. If nobody knows it, we have to measure it by ourselves. If all the knowledge needed are too much, we have to collaborate with others. [http://en.wikipedia.org/wiki/Computer-aided_design Computer-aided design] have to be used to accomplish more complex tasks. [http://en.wikipedia.org/wiki/Space_Shuttle Space shuttle] and [http://en.wikipedia.org/wiki/Microprocessor microprocessor] are seen as the most complex systems engineered, but they are far too simple when compared with the [http://en.wikipedia.org/wiki/Complexity complexity] of biological systems created by evolution.
On the other side, [http://en.wikipedia.org/wiki/Evolution evolution] is a completely different process. Variation is random, selection is directional based on the fitness to the environment, that is all. However, all the amazing things on our planet is emerged from this simple process, no more thing is needed.
Why? All the complexity is solved by this simple process without any input of knowledge?
Yes. The answer is on the scale. The evolution process is so simple that it can be scaled up infinitely. The complex problem can be solved in the distributed evolution system. Any success in the distributed system can be amplified by the selection process. Therefore, although the variation is random, it will be powerful enough to search for the solutions, when the scale of the system is big enough.
In the design process, the variation is directional based on the knowledge, but the process is not scalable, because of its requirement of intelligence. As a result, it is much more difficult to solve complex problems by design.
Directed Evolution
Although Darwin's theory was published 150 years ago, people have used the power of evolution more than ten thousand years. [http://en.wikipedia.org/wiki/Domestication Domestication] of plants and animals is done by [http://en.wikipedia.org/wiki/Artificial_selection artificial selection], which is the process of intentional breeding for certain traits, therefore changing the direction of evolution.
[http://en.wikipedia.org/wiki/Directed_evolution Directed evolution] is a method using the similar principle to create new biomolecules with desired properties. The targets of directed evolution include enzymes, antibodies, [http://en.wikipedia.org/wiki/Aptamer aptamer]s, [http://en.wikipedia.org/wiki/Ribozyme ribozyme]s, biosynthetic pathways, and synthetic genetic circuits. More information can be found on [http://www.che.caltech.edu/groups/fha/home.html the Frances H. Arnold research group], [http://ellingtonlab.org the Ellington lab], and [http://genetics.mgh.harvard.edu/szostakweb/ the Szostak lab].
The directed evolution experiment contains several rounds of 3 steps: variation, selection, and amplification. Variation is the mutation or recombination of the information encoded in the DNA, usually by error-prone PCR and [http://en.wikipedia.org/wiki/DNA_shuffling DNA shuffling] respectively. Selection is the process of separating the variants with desired phenotypes from others, it can either refer to screening (isolate good variants) or selection (eliminate bad variants), either in vivo or in vitro. Amplification is the replication of the variants after selection, which recovers the population size for the new round of directed evolution.
New function of biomolecules or biological systems is difficult to be rationally designed, but directed evolution is successfully used in solving these problems.
Design & Evolutionary Approaches in iGEM Projects
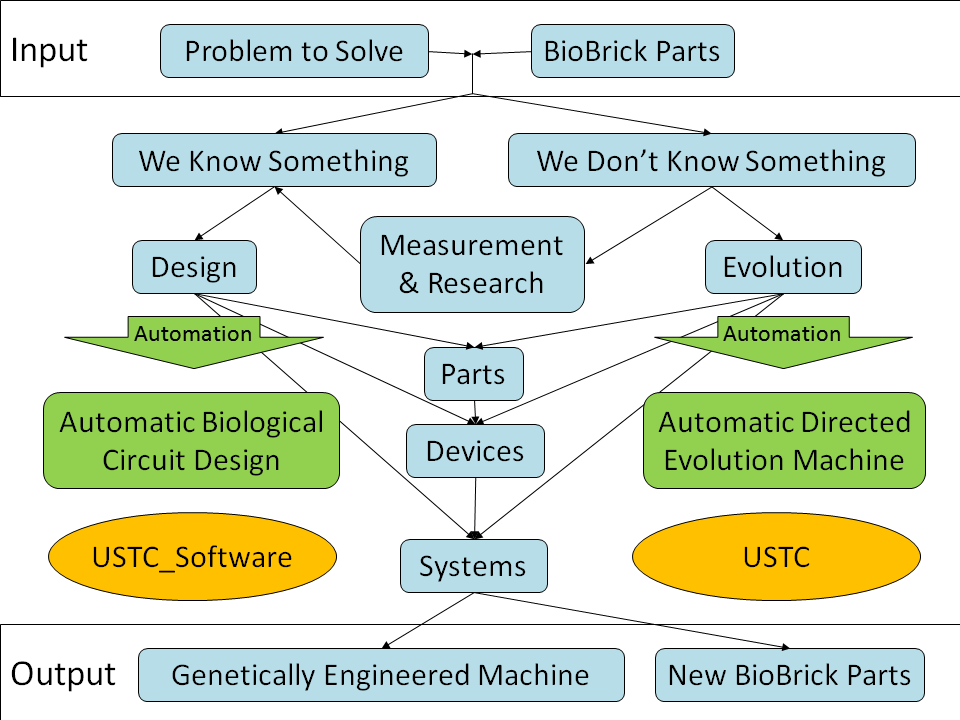
In the iGEM competition, teams specify, design, build, and test simple biological systems made from standard, interchangeable biological parts. Most projects engineer the systems by modeling and measurement, including this project itself. This engineering approach is proved to be feasible but difficult in the design of biological systems during this years.
Most BioBrick parts have never been characterized, because characterization and documentation of the parts usually takes a lot of time. Further more, the properties of BioBrick parts and devices are very sensitive to the conditions they work in, such as strain, plasmid, other parts used together, culture medium, growth rate, temperature, pH, shaking rate, light, and so on. It is very difficult to characterize all the parameters, so the parts often have to be remeasured in different projects before the behavior of the system can be predicted.
There are also many projects using evolutionary approaches. These approaches use selection or screening to find the needed part, eliminate the steps of measurement or constructing new parts with desired properties.
| Team Name / People | Competition / Lab | Project | Approach | Wiki Link |
|---|---|---|---|---|
| Davidson | iGEM 2006 | Solving the Pancake Problem with an E. coli Computer | in vivo Recombination; Selection | [http://parts2.mit.edu/wiki/index.php/Davidson_2006 Davidson] |
| Missouri_Western | iGEM 2006 | Solving the Pancake Problem with an E. coli Computer | in vivo Recombination; Selection | [http://parts2.mit.edu/wiki/index.php/Missouri_Western_State_University_2006 Missouri_Western] |
| Alberta | iGEM 2007 | Plan B: Building Butanol BioBricks | Mutagenic Compound; Selection | [http://parts.mit.edu/igem07/index.php/Alberta Alberta] |
| Boston_University | iGEM 2007 | Increasing the Current Output of Microbial Fuel Cells Through the Directed Evolution of Shewanella oneidensis | Error-Prone PCR; Screening | [http://parts.mit.edu/igem07/index.php/Boston_University Boston_University] |
| Davidson_Missouri_W | iGEM 2007 | Living Hardware to Solve the Hamiltonian Path Problem | in vivo Recombination; Screening | [http://parts.mit.edu/igem07/index.php/Davidson_Missouri_W Davidson_Missouri_W] |
| Harvard | iGEM 2007 | Cling-E. coli: Bacteria on target | Screening | [http://parts.mit.edu/igem07/index.php/Harvard Harvard] |
| USTC | iGEM 2007 | Extensible Logic Circuit in Bacteria | Error-Prone PCR; Screening | [http://parts.mit.edu/igem07/index.php/USTC USTC] |
| Calgary_Software | iGEM 2008 | EvoGEM: An Evolutionary Approach to Designing Life | Evolutionary Algorithm | Calgary_Software |
| ETH_Zurich | iGEM 2008 | Make yourself simpler, stupid! Or how engineering a self-minimizing cell leads to the Minimal Genome | Controlled expression of restriction enzymes and ligases in vivo; Selection | ETH_Zurich |
| IIT_Madras | iGEM 2008 | StressKit: A BioBrick library of Lac-repressed σ24, σ28, σ32 and σ38 promoters for Escherichia coli | Screening | IIT_Madras |
| Illinois | iGEM 2008 | Cell-based and in vitro antigenic sensors for medical diagnostics | Error-Prone PCR; Screening | Illinois |
| Newcastle_University | iGEM 2008 | A Computational Intelligence Approach to Developing a Diagnostic Biosensor: The Newcastle BugBusters Project | Evolutionary Algorithm | Newcastle_University |
| Peking_University | iGEM 2008 | A genetic circuit to direct evolution of proteins in vivo | in vivo Mutagenesis; Screening | Peking_University |
| Tokyo_Tech | iGEM 2008 | Coli.Touch – implementation of a pressure-responsive genetic circuit in E. Coli | Error-Prone PCR; Screening | Tokyo_Tech |
| Warsaw | iGEM 2008 | Bacterial device for creating and production of interactors for any given bait protein | in vivo Mutagenesis; Selection | Warsaw |
| Jason R. Kelly | Endy Lab | Screening plasmid | Screening | [http://openwetware.org/wiki/Endy:Screening_plasmid Endy:Screening_plasmid] |
Evolutionary Biology, Population Genetics & Evolutionary Algorithm
The process of evolution is as simple as the repeat of variation and selection, but it is also more complex than everything we can imagine. While an organism is evolving to fit the environment, the environment containing other organisms are also evolving. The variation rate of the organism and the selection pressure of the environment themselves are also evolving to keep the survival of the entire ecosystem.
There are many theoretical researches of evolution following the work of Darwin. [http://en.wikipedia.org/wiki/Portal:Evolutionary_biology Evolutionary biology], [http://en.wikipedia.org/wiki/Population_genetics population genetics] and [http://en.wikipedia.org/wiki/Evolutionary_algorithm evolutionary algorithm] are 3 research fields that are closely related to the research of the dynamics of evolution. The first two fields are on the biology side, while evolutionary algorithm is an engineering approach widely used in optimization problems.
[http://en.wikipedia.org/wiki/Fitness_landscape Fitness landscape] is often used to characterize evolution. Mountain peaks are high ability to survive, while valleys represent low fitness. Similar genotypes are close to each other, while different ones are far from each other. The population takes an adaptive walk across such a landscape. The paths of evolution is determined by the fitness landscape.
Problems to Be Solved
Evolutionary algorithm have been used for more than 50 years, many strategies have been and are being developed to improve the efficiency of the algorithm. These strategies deal with the control of evolution parameters. There are deterministic control, adaptive control (feedback control), and self-adaptive control (the evolution of evolution).
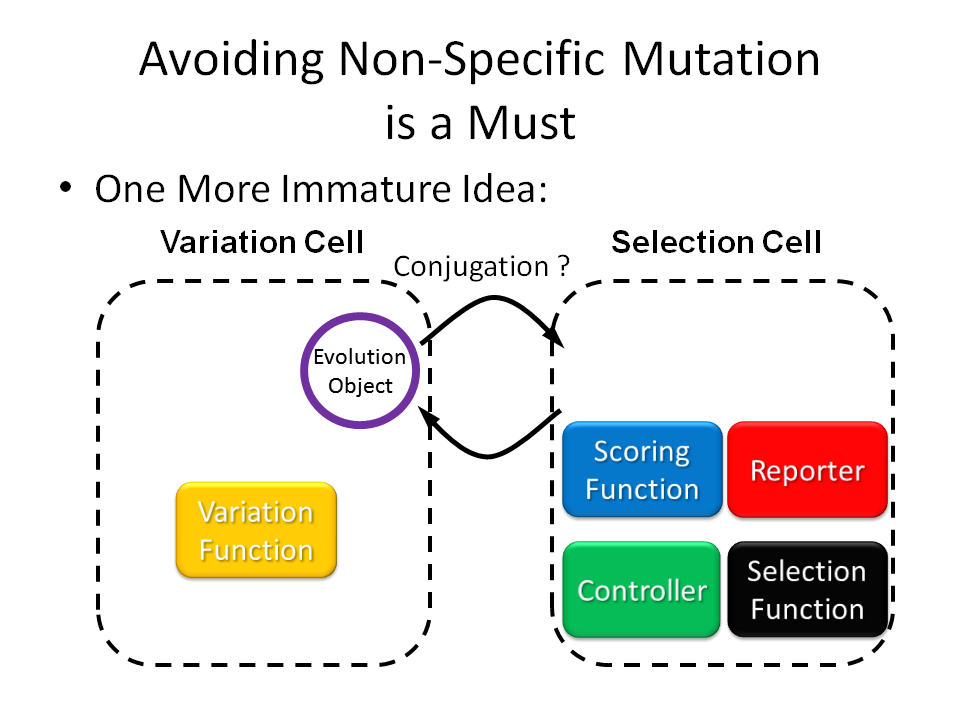
Directed evolution experiments are usually carried out by hand, the parameters are optimized according to experience. Therefore, it will take a lot of time to optimize conditions, especially for new targets. When the condition is not optimized, different fitness may not be well separated, and the success rate will be low.
If the conditions can be controlled automatically according to theories of evolutionary algorithm, it is expected to greatly increase the success rate and reduce the time, labor and cost. However, automation of the experiments is not practicable under today's technology.
Fortunately, the rapid development of synthetic biology has opened another possibility. Biological systems become programmable now. It is possible to automate the process in the cell, to make an E. coli cell an automatic directed evolution machine.
The Blueprint
The Goal
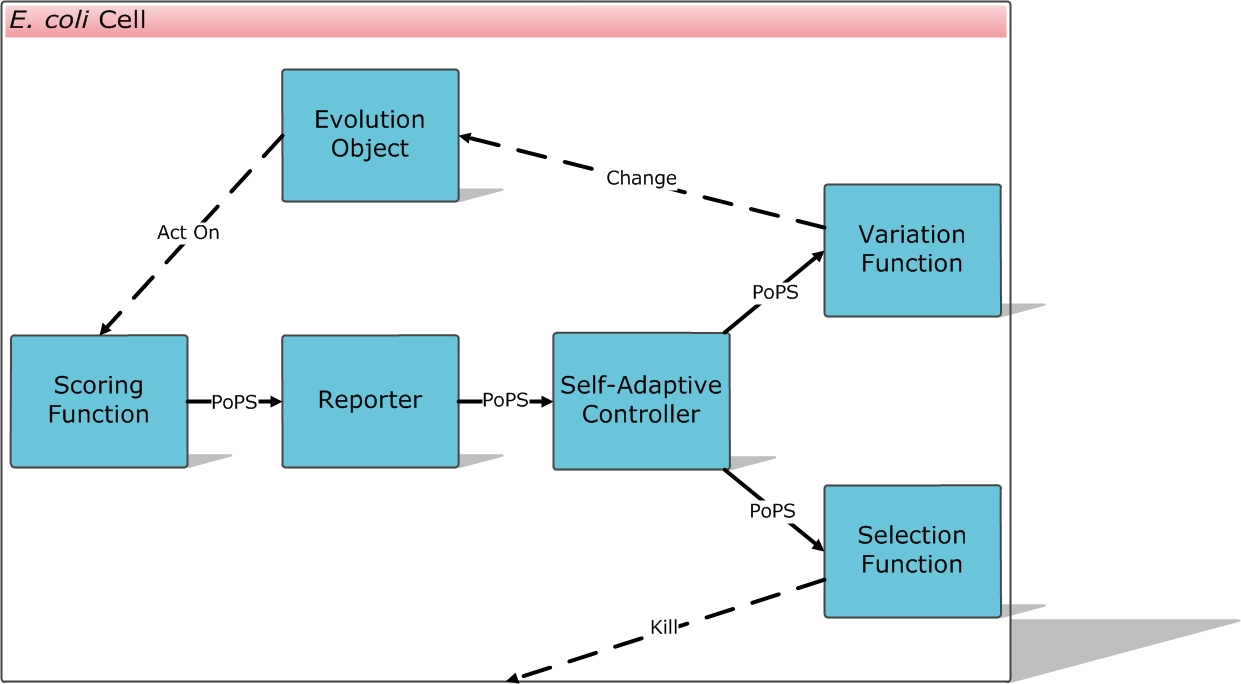
We designed the E. coli Automatic Directed Evolution Machine (E.ADEM) project.
The ultimate goal is to make E.ADEM a universal framework for evolutionary approaches in synthetic biology. Anything we want in synthetic biology can be automatically created, from promoters, RBS, regulators, receptors, binding partners, aptamers, enzymes and ribozymes, to sensors, logic devices, reporters, metabolic pathways, entire genomes, and even solutions of mathematic problems.
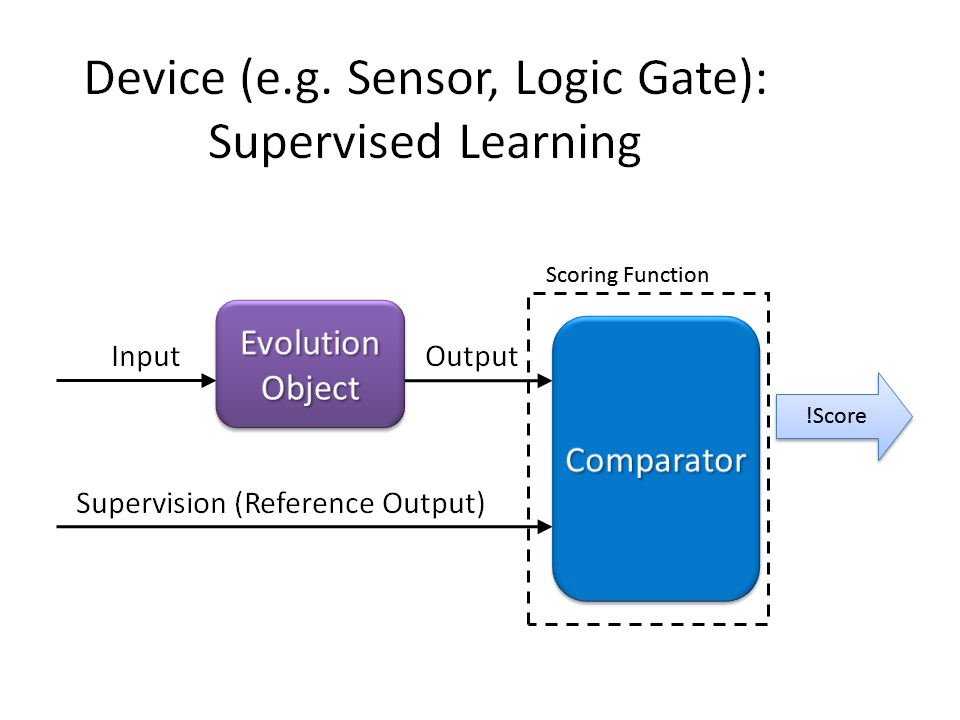
To each evolution object you want it to evolve, a scoring function can be designed to output PoPS as the fitness score to your demand. After that, you can ligate the scoring function device into the E.ADEM plasmid, transform E. coli, culture the cells and wait for them to evolve automatically and robustly, and get what you want at last.
Modules & Flow Chart of the System
Scoring Function
Possible Design:
For Transcription Repressor:
For Device (e.g. Sensor, Logic Gate):
For Enzyme:
If there is a sensor for the product or substrate, use it as the scoring function.
Else, perform evolution for a sensor first.
For Binding Partner:
Use E. coli two-hybrid systems.
Self-Adaptive Controller
Function: adjust variation rate and selection pressure, base on the fitness score, the population size and the average fitness score calculated by a quorum sensing device.
Problems:
- Variation rate
- Too low: Slow
- Too high: Misconvergence or Death
- Selection pressure
- Too low: Nondirectional
- Too high: Die out
See below for our design.
Variation Function
Possible Design:
- Targeted Mutagenesis
- Activation induced cytidine deaminase (AID)
- iGEM 2008 Peking_University
- iGEM 2008 Warsaw
- GM Church method GM Church method
- Error-prone DNA polymerase I
- Bacteriophage ?
- Error-prone reverse transcription ?
- Recombination
- Site-specific recombination (including flip, insert and delete)
- Homologous recombination
<biblio>
- GM Church method pmid=19633652
</biblio>
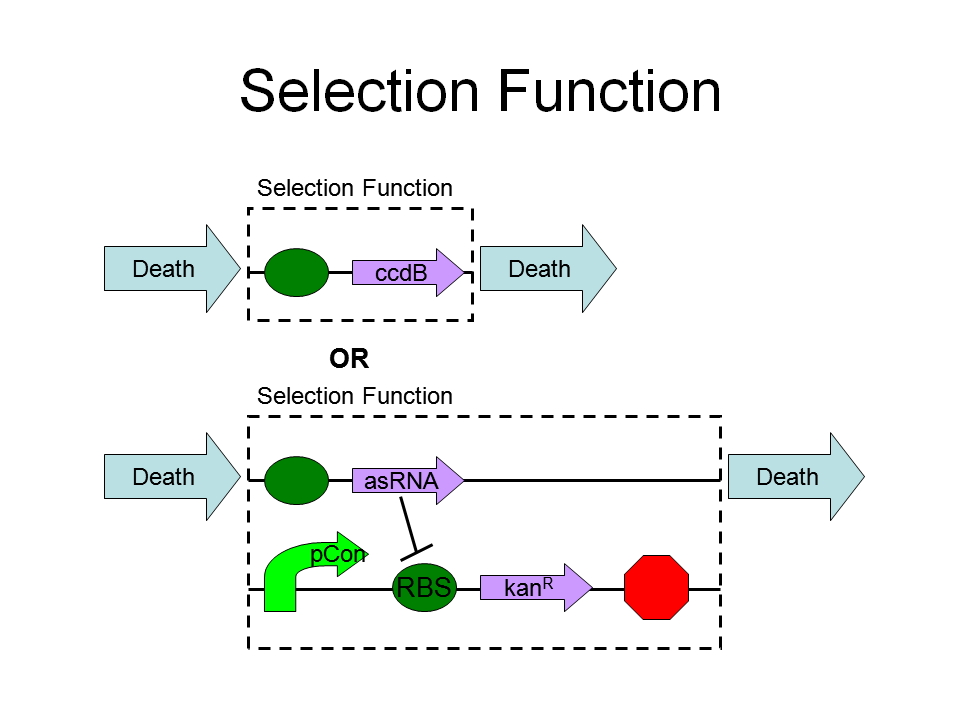
Selection Function
Kill or survive the cell:
Repoter
The Prototype
What to Do First?
The Self-Adaptive Controller is the core of the machine. We must finish it first in the prototype machine.
The Selection Function is important to test the Self-Adaptive Controller.
The Variation Function can be made later.
The Scoring Function can be made later. Constitutive Promoter Family can be used as Stimulus Signals for the Self-Adaptive Controller.
Constitutive Promoter Family as Stimulus Signals
We choose to use constitutive promoters instead of the conditional operon impressions (represented by [http://en.wikipedia.org/wiki/Lac_operon IPTG-induced expression]) as the stimulus signals to test the system. The stimulus signals in a testing system are supposed to be definite and stable. However, the IPTG-induced signals are susceptible to many environmental factors. The process of inducible expression involves a series of dynamic actions in physical chemistry: the diffusion process of IPTG molecules, and the equilibruim between the attachment and disattachment of IPTG to the promoter. That way, the expression signals would fluctuate in a large scope in experiments and the mathematic analyses would be very complicate.
Comparatively, the stimulus signals based on a series of constitutive promoters of different levels are far stabler since the process are relatively direct.
- The signals produced by the constitutive promoters can maintain at the steady state during the measurement.
- Constitutive-promoter expressions can give out several different stimulus signals in one system without any disturbation among them. That is, several constitutive promoters can work independently in one system to produce double or triple stimulus signals. Instead, the IPTG-induced testing system can imput only one signal at a time corresponding to the concentration of the IPTG.
- Two or more systems with different stimulus signals can grow in the same nurture if the signals are produced by the constitutive promoters. For example, in our project, several kinds of E.coli with different imputs signals can grow in the same culture medium preparing for the screen.
Design of the Self-Adaptive Controller
|
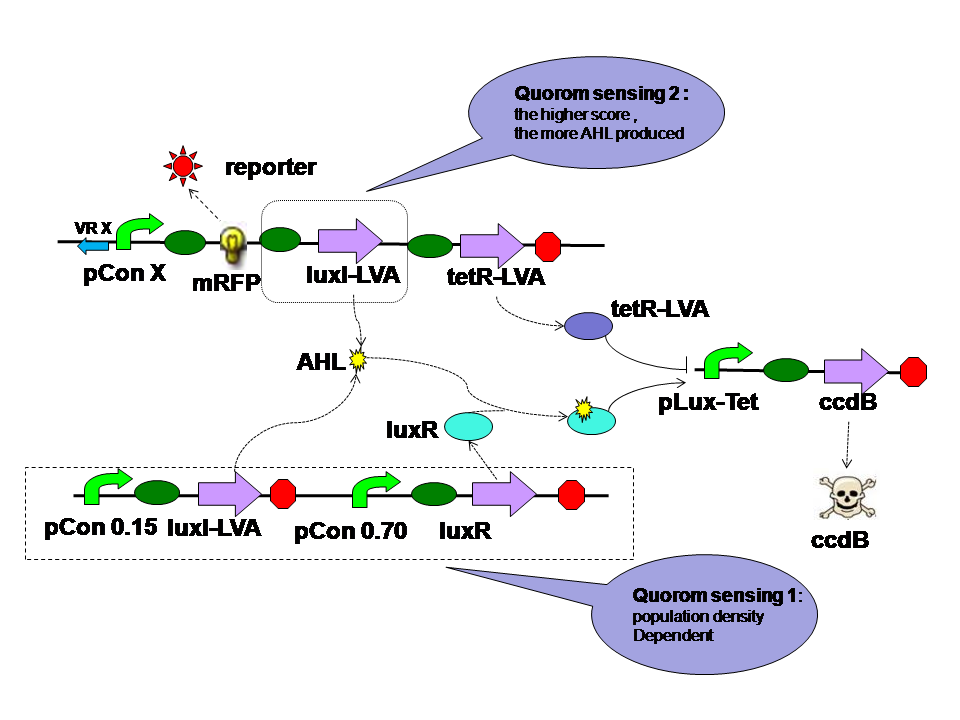
The self-adaptive controller consists of two quorom sensing parts one tetR parts and a hybrid promoter. |
|
There are two quorom sensing parts in the self adaptive controller. LuxR is expressed by a constitutive promoter and interacts with AHL produced by both LuxI in two parts. The complex then activates hybrid promoter. LuxI in different parts perform different functions. LuxI's expression in Quorom sensing 1 depends on the population density and take effect in evolution in the aspect of cell density. LuxI in part 2, on the other hand, is expressed correspondent to the evolution score directly. Thus it takes part in the evolution process directly. |
|
TetR is expressed correspondent to the evolution score, just like the LuxI from quorom sensing part 2. However tetR represses the hybrid promoter. High score means high tetR level. High tetR level means low death rate. |
Principle of Operation
- Properties of selection system
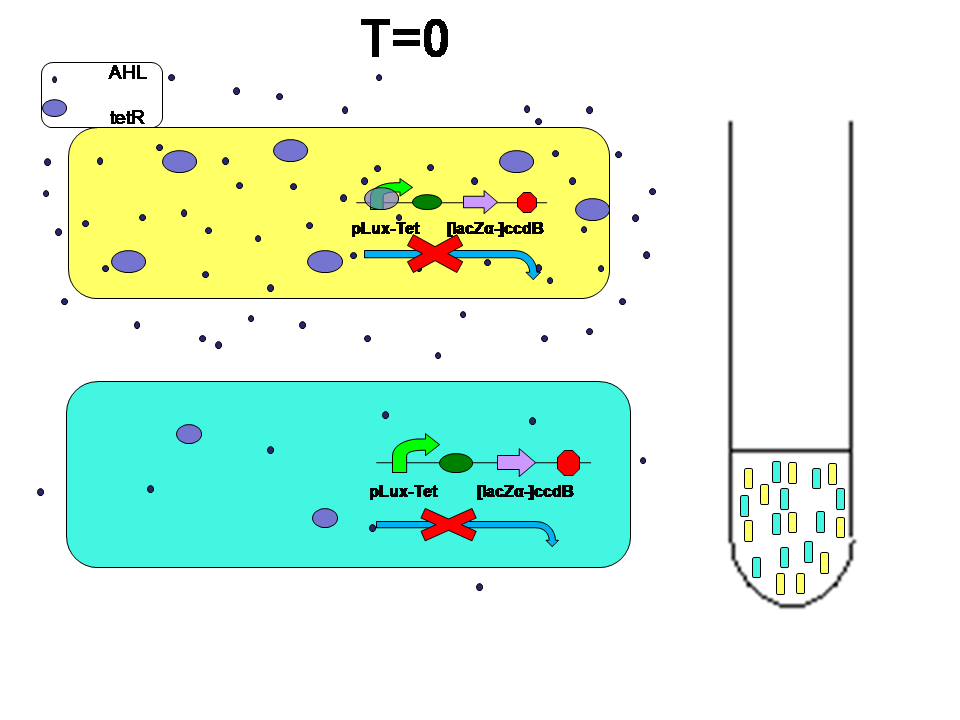
The selection system consists of hybrid promoter and ccdB. AHL-LuxR complex and tetR input perform contrast effect. We perform several experiments to identify how the hybrid promoter work. The property is as shown below:
- How different "scores" result in evolution
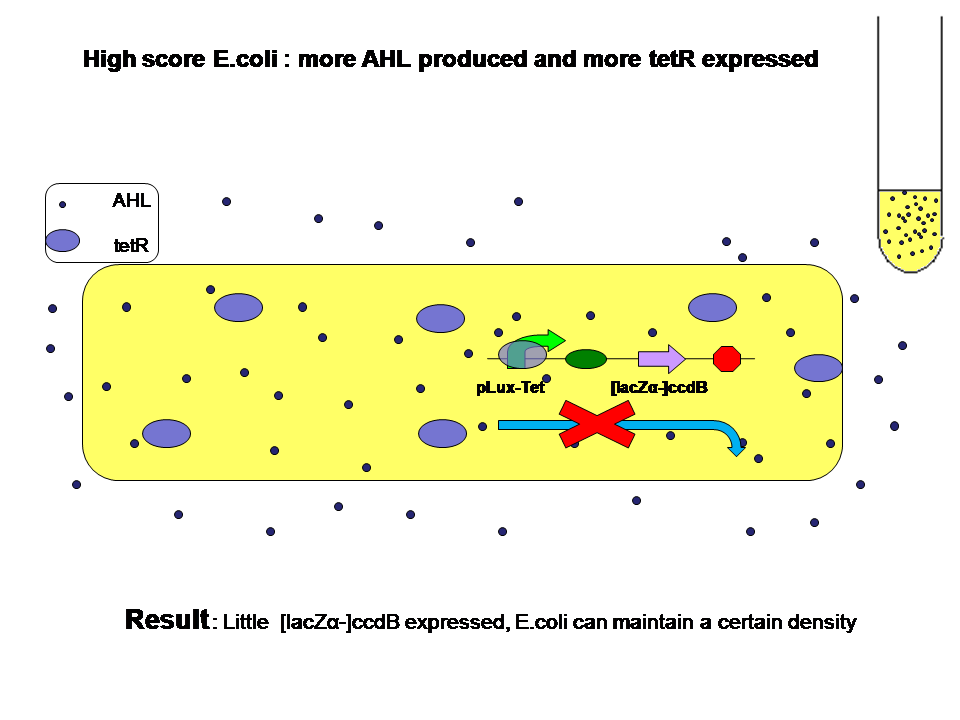
When high score and low score E.coli are grown seperately:
Low score produce little AHL and little tetR. High score produce more AHL and more tetR at the same time. Two kinds of E.coli will survive in their own way. They will both reach high population density.
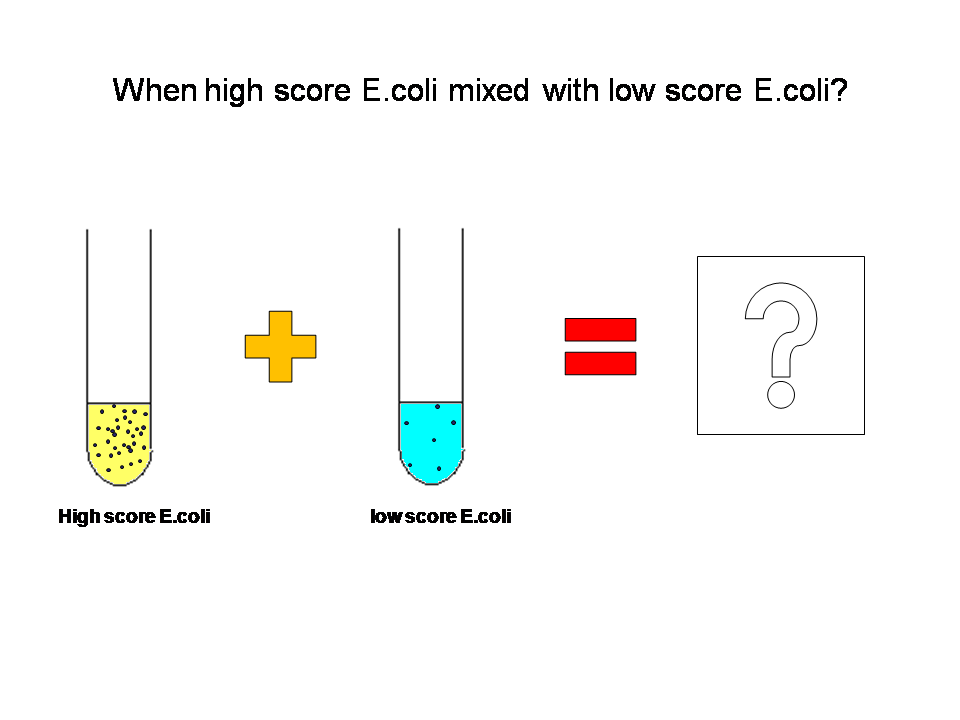
What if we mix them together?
At first, two kinds of E.coli were mixed 1:1. Two kinds of E.coli maintain their own density state.
Average AHL level begin to change.Low score E.coli in the high level AHL circumustance will express more ccdB. Then the high score E.coli has a advantage over the low score.More high score ones will survive. The difference between score finally results in the evolution.
Vector & Chassis
Vector:
PSB1A3: high copy number, therefore less sensitive to mutation.
Chassis:
Top10: common used strain.
MDS™ 42 recA Blue:
http://www.scarabgenomics.com/
Scarab Genomics has bioengineered the Clean Genome® E. coli by deleting over 15% of the E. coli K-12 genome. Using synthetic biology methods, the K-12 genome was rationally designed by making a series of precise deletions, which included the elimination of non-essential genes, recombinogenic or mobile DNA, and cryptic, virulent genes. This genome reduction optimizes the E. coli strain as a biological factory, providing enhanced genetic stability and improved metabolic efficiency. These properties make the Clean Genome® the E. coli strain of choice for a wide spectrum of applications ranging from routine cloning to production of biological material for therapeutic purposes.
Scarab Genomics was established in 2002. The Clean Genome® E. coli is the result of Dr. Fred Blattner's research at the University of Wisconsin, Madison. Scarab Genomics has licensed the patented reduced genome technology from Wisconsin Alumni Research Foundation on an exclusive, worldwide basis.
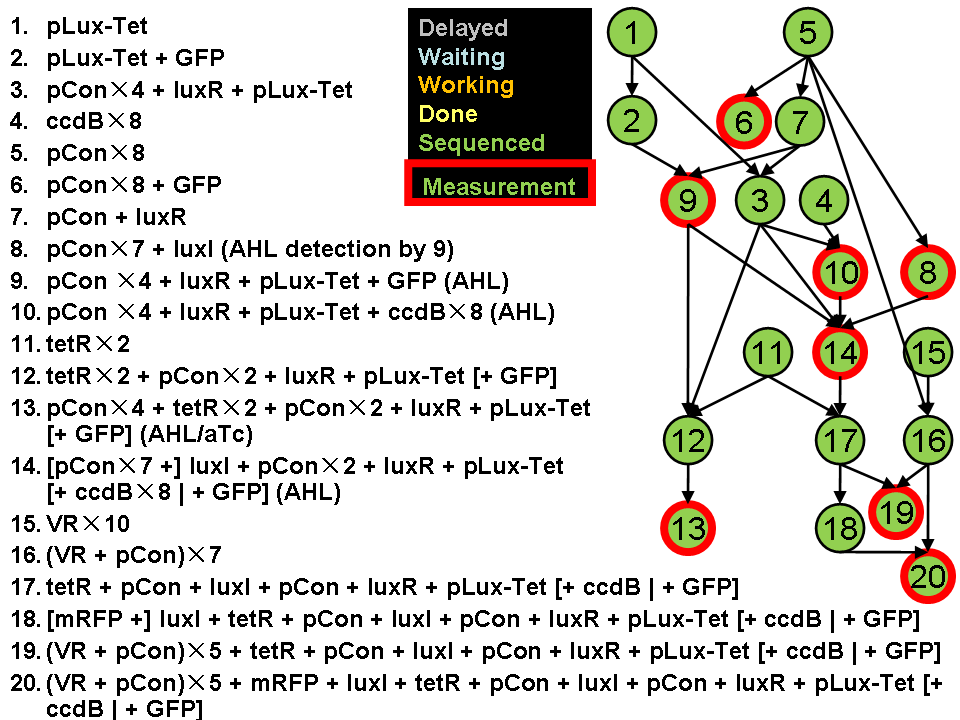
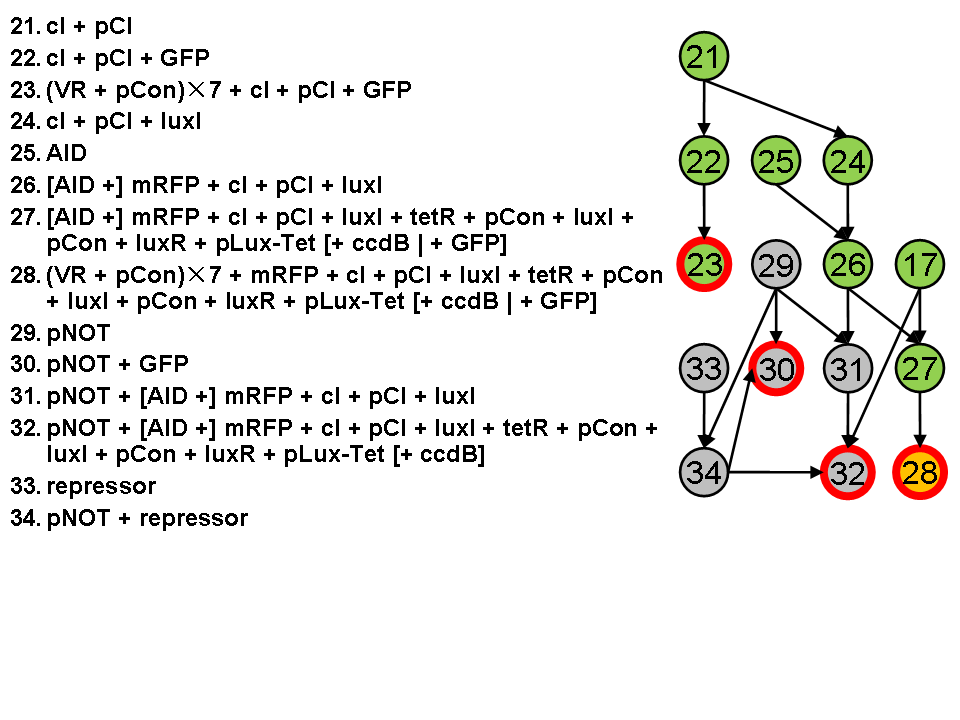
Assembly Road Map
In order to keep the whole process in perspective, we designed maps to direct our work in wet lab.
The Progress
- Assembly work
As shown in the roadmap of our work, we finished assemblying most of the designed system except the pNOT parts. We submitted 167 biobricks finally.
- Measument work : Most our parts are measured to prove their utility and get useful parameters.
- 6 constitutive promoter are measured and marked with barcode system.
- Hybrid promoter and related tetR,LuxI,LuxR are proven to work well in our models.
- Several models were established and fitted our data pretty well.
- Density control parts(ccdB related) do not work stably and need further improvements.We tried to add LVA to ccdB to improve its stability ,but it did not work.
Beyone iGEM
http://openwetware.org/wiki/Synthetic_Biology http://openwetware.org/wiki/Synthetic_Biology:Collaborative_Projects http://openwetware.org/wiki/E._coli_Automatic_Directed_Evolution_Machine_project
 "
"