Team:Valencia/WetLab/YeastTeam/Results
From 2009.igem.org
| (46 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:Valencia09iGEM23}} | {{Template:Valencia09iGEM23}} | ||
| + | <html> | ||
| + | <style> | ||
| + | #content{ | ||
| + | height: 4200px; | ||
| + | } | ||
| + | </style> | ||
| + | </html> | ||
| - | <div style="position:relative; top:- | + | |
| + | <div style="position:relative; margin-top:-295px; margin-left:190px; width:700px; font-family: Verdana;" align="justify"> | ||
<br> | <br> | ||
| - | = | + | ='''Experimental results'''= |
| - | + | ||
<br> | <br> | ||
| - | |||
Our ultimate goal was to make a bio-screen made with cellular pixels (LECs). But, before to be able to build this iLCD, we had to study the behaviour of one single LEC. Therefore, we focused on the electrical excitation of our transformed yeasts. | Our ultimate goal was to make a bio-screen made with cellular pixels (LECs). But, before to be able to build this iLCD, we had to study the behaviour of one single LEC. Therefore, we focused on the electrical excitation of our transformed yeasts. | ||
| + | This is the behaviour that stands as the cornerstone of our project. '''With a serie of voltage inputs, we accomplished a series of light emissions'''. No exciting light was needed, only aequorin, coelenterazine (the prosthetic group) and Ca<sup>2+</sup>. | ||
| - | + | <table> | |
| + | <tr> | ||
| + | <td>[[Image:Valencia_Grafica_continu_2_pics.jpg|500 px|left]]</td> | ||
| + | <td>[[Image:Bursts.gif|100 px|center]]</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | Arrows indicate when voltage was applied: 16V during 5 seconds applied at 300 seconds (5 minutes) and 600 seconds (10 minutes). Movie on the right shows how the response would be seen in a pixel. | ||
| + | *'''WT''' indicates our LEC: our aequorin-engineered yeast, with coelenterazine and wild-type calcium channels | ||
| + | *'''WT -coe''' indicates the same yeast, without the addition of coelenterazine. | ||
| - | + | As we can observe, '''only our LEC is excited when electric current is applied'''. Withour coelenterazine there is no response to that stimulus. | |
| - | + | ||
| - | == | + | ==Conclusions of the study== |
<br> | <br> | ||
| - | + | We have investigated a set of '''different voltages and times in order to precisely know how our system respons to electric current. Knowing this, we could control it properly. | |
| - | [[ | + | We will show and explain here the '''major results''', for a thorough description of the behaviour of the aequorin light emission system please refer to the [https://2009.igem.org/Team:Valencia/Parts/Characterization '''Characterization page'''] or to the [http://partsregistry.org/wiki/index.php?title=Part:BBa_K222000 '''Registry''']. |
| + | <br> | ||
| + | ===Controls=== | ||
| + | <br> | ||
| + | In every single experiment in a molecular biology laboratory, one has to bear in mind the use of negative controls for every logical step of the hypothesis. | ||
| + | Therefore, we have taken advantage of the different experimental designs that were available: we had several calcium channel knock outs (''mid1'' and ''ch1''), as well as functional inhibitors of the calcium channels (KCl) and divalent ion quelant (EDTA). | ||
| - | + | All these designs were used in order to reject the idea of looking to an artifact. | |
| - | + | [[Image:Comparació_disc.jpg|700 px||left]] | |
| - | + | This graph shows the behaviour of a set of designs after the supply of a 4V shock. | |
| - | * ''' | + | * '''WT''' is our wild type luminiscent cell, with coelenterazine and fully working calcium channels |
| - | * ''' | + | * '''mid1''' is a knock out for a calcium channel. Light is not observed because Ca2+ can’t enter into the cell and bind to the aequorin-coelenterazine complex. |
| + | * '''cch1''' is another knock out mutant for a calcium channel, so the absence of light can be explainned in the same way. | ||
| - | + | * '''EDTA''' is a divalent ion quelant, so Ca2+ is quenched and not useful for the light emission, although every compound necessary for the reaction is present. | |
| - | + | ||
| - | + | * '''SD +coe''' is growth media with coelenterazine, just to be sure that without cells we had no light. | |
| - | + | * '''SD''' is plain growth media. | |
| - | + | * '''yeast -coe''' is our wild type luminiscent cell, without coelenterazine, the prosthetic group that is needed for the light emission. | |
| + | Three kinds of behaviour stem out of this experiment: no light at all, some light emission and full light emission. | ||
| + | * ''no light at all'' is the case of '''SD +coe''','''SD''' and '''yeast -coe''' where there is a lack of at least one of the compnents of the LEC. | ||
| + | * ''some light emission'' is the case of '''mid1''', '''cch1''' and '''EDTA''' where all the LEC components are present, but free calcium in the cell is somehow constrained. | ||
| + | * ''full light emission'' is the case of '''WT''' where all the components of the LEC are presents and calcium channels are fully open. | ||
| + | <br> | ||
| + | ===Voltage=== | ||
| + | <br> | ||
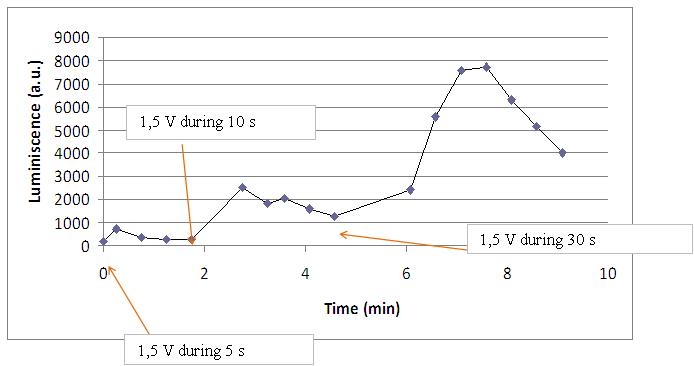
| + | Varying voltage and exciting time, we can have a variety of responses (arrows indicate electric current supply): | ||
| + | <center> | ||
| + | {| | ||
| + | |[[Image:1,5V 10s.jpg|center|450px]] | ||
| + | |- | ||
| + | |[[Image:4,5V 5s.jpg|center|450px]] | ||
| + | |} | ||
| + | </center> | ||
| - | + | ===Supply time=== | |
| + | <br> | ||
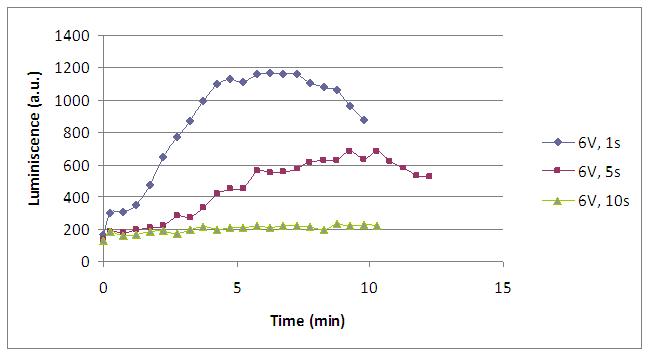
| + | Varying the electric current time supply we can study the influence of this variable in our system. Here we see that if we apply too much time the system is overdosed. Figure on the right shows the behaviour of the three pixels, the third one would be a blow pixel as a consequence of an oversupply of voltage. | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td>[[Image:6V variats disc.jpg|410px|center]]</td> | ||
| + | <td>[[Image:Sixvolts.gif|360 px|center]]</td> | ||
| + | </tr> | ||
| + | </table> | ||
| - | + | ===Repetition=== | |
| + | <br> | ||
| + | Our idea was to have a screen, that is '''to have several images sequentially''', in order to see animated pictures. So we studied the different voltage times in a sequence of power applications to one LEC. | ||
| + | [[Image:Manteniment resposta disc.jpg|center|600px]] | ||
| - | === | + | ===Refreshing rate=== |
<br> | <br> | ||
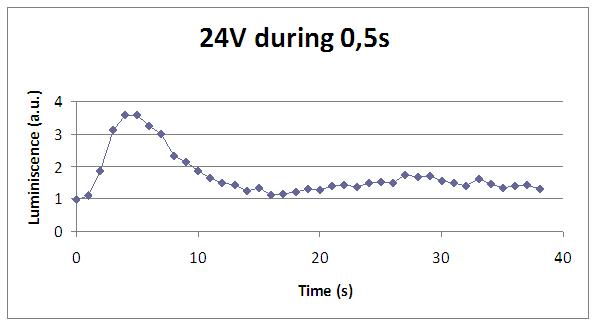
| - | + | Finally, we were looking for the shortest refreshing rate as possible, so to have something close to a ''real'' screen. | |
| - | + | [[Image:24V 0,5s.jpg|center|500 px]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[Image:24V 0,5s.jpg|center| | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | We have accomplished a refreshing rate of 12 seconds with a supply of 24V in 0,5 seconds. | |
| - | |||
| - | + | Again, if you find yourself hungry of knowledge, please find much more information on the aequorin and the study we have made upon it on the [https://2009.igem.org/Team:Valencia/Parts/Characterization '''Parts Characterization'''] page or on the [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2009&group=Valencia&Done=1 '''Registry''']. | |
| - | + | ||
Latest revision as of 03:13, 22 October 2009
Experimental results
Our ultimate goal was to make a bio-screen made with cellular pixels (LECs). But, before to be able to build this iLCD, we had to study the behaviour of one single LEC. Therefore, we focused on the electrical excitation of our transformed yeasts.
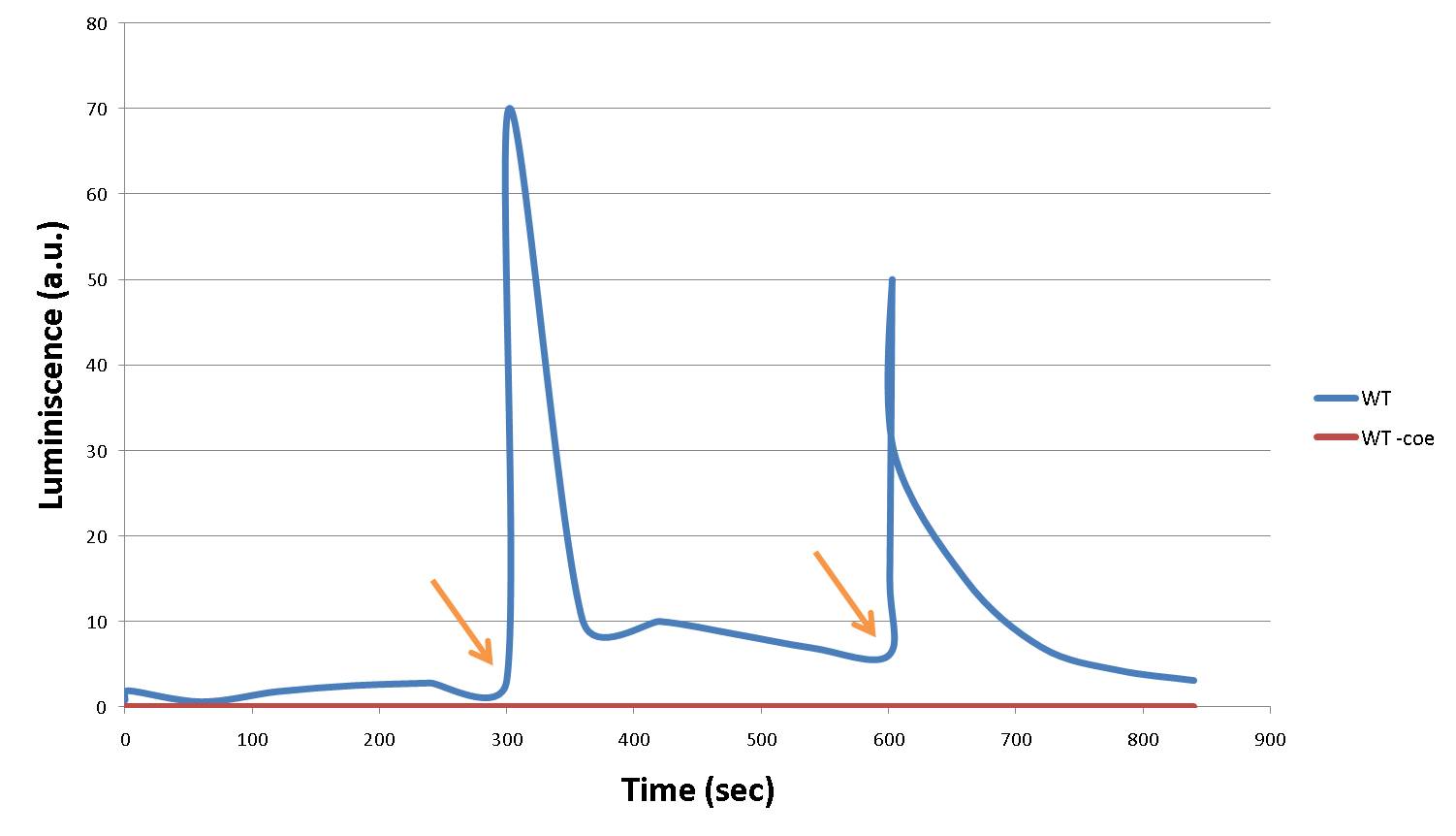
This is the behaviour that stands as the cornerstone of our project. With a serie of voltage inputs, we accomplished a series of light emissions. No exciting light was needed, only aequorin, coelenterazine (the prosthetic group) and Ca2+.
Arrows indicate when voltage was applied: 16V during 5 seconds applied at 300 seconds (5 minutes) and 600 seconds (10 minutes). Movie on the right shows how the response would be seen in a pixel.
- WT indicates our LEC: our aequorin-engineered yeast, with coelenterazine and wild-type calcium channels
- WT -coe indicates the same yeast, without the addition of coelenterazine.
As we can observe, only our LEC is excited when electric current is applied. Withour coelenterazine there is no response to that stimulus.
Conclusions of the study
We have investigated a set of different voltages and times in order to precisely know how our system respons to electric current. Knowing this, we could control it properly.
We will show and explain here the major results, for a thorough description of the behaviour of the aequorin light emission system please refer to the Characterization page or to the [http://partsregistry.org/wiki/index.php?title=Part:BBa_K222000 Registry].
Controls
In every single experiment in a molecular biology laboratory, one has to bear in mind the use of negative controls for every logical step of the hypothesis.
Therefore, we have taken advantage of the different experimental designs that were available: we had several calcium channel knock outs (mid1 and ch1), as well as functional inhibitors of the calcium channels (KCl) and divalent ion quelant (EDTA).
All these designs were used in order to reject the idea of looking to an artifact.
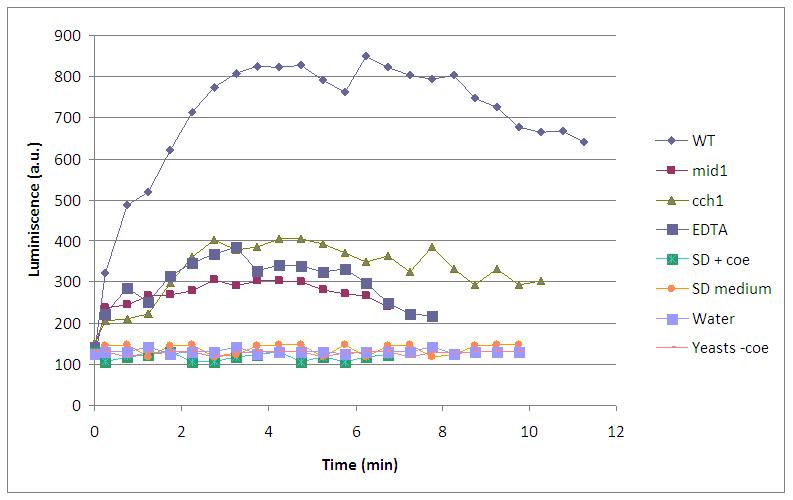
This graph shows the behaviour of a set of designs after the supply of a 4V shock.
- WT is our wild type luminiscent cell, with coelenterazine and fully working calcium channels
- mid1 is a knock out for a calcium channel. Light is not observed because Ca2+ can’t enter into the cell and bind to the aequorin-coelenterazine complex.
- cch1 is another knock out mutant for a calcium channel, so the absence of light can be explainned in the same way.
- EDTA is a divalent ion quelant, so Ca2+ is quenched and not useful for the light emission, although every compound necessary for the reaction is present.
- SD +coe is growth media with coelenterazine, just to be sure that without cells we had no light.
- SD is plain growth media.
- yeast -coe is our wild type luminiscent cell, without coelenterazine, the prosthetic group that is needed for the light emission.
Three kinds of behaviour stem out of this experiment: no light at all, some light emission and full light emission.
- no light at all is the case of SD +coe,SD and yeast -coe where there is a lack of at least one of the compnents of the LEC.
- some light emission is the case of mid1, cch1 and EDTA where all the LEC components are present, but free calcium in the cell is somehow constrained.
- full light emission is the case of WT where all the components of the LEC are presents and calcium channels are fully open.
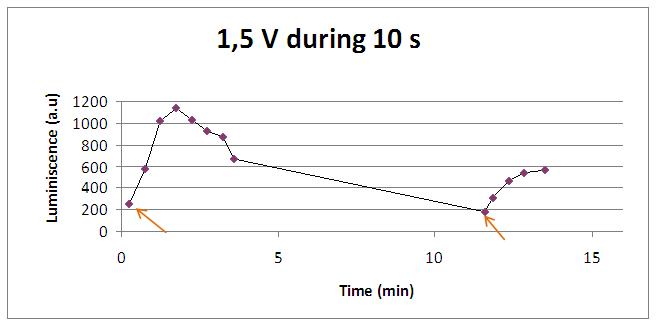
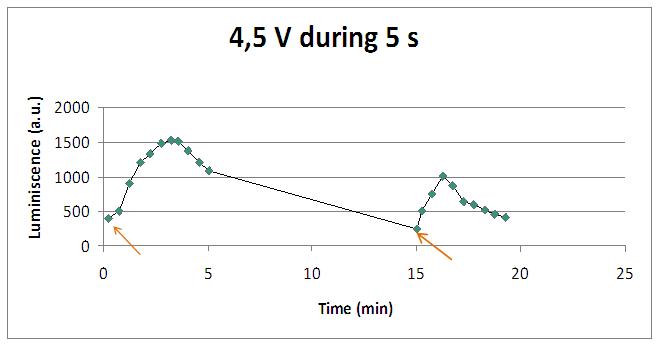
Voltage
Varying voltage and exciting time, we can have a variety of responses (arrows indicate electric current supply):
Supply time
Varying the electric current time supply we can study the influence of this variable in our system. Here we see that if we apply too much time the system is overdosed. Figure on the right shows the behaviour of the three pixels, the third one would be a blow pixel as a consequence of an oversupply of voltage.
Repetition
Our idea was to have a screen, that is to have several images sequentially, in order to see animated pictures. So we studied the different voltage times in a sequence of power applications to one LEC.
Refreshing rate
Finally, we were looking for the shortest refreshing rate as possible, so to have something close to a real screen.
We have accomplished a refreshing rate of 12 seconds with a supply of 24V in 0,5 seconds.
 "
"