Team:TUDelft/13 August 2009
Lab Notebook
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
13 August 2009
Sriram
Today i understood that running the gel with ligated products is unnecessary which is clear in the below gel. I must not do this mistake again. I plated the transformed colonies on the plate.
Calin
Knockout day. [http://openwetware.org/wiki/NanoBio:_Protocol_for_gene_knockout NanoBio: Protocol for gene knockout]
Made 1M l-arabinose stock. 150mg into 0.85 mL ddH20.
Digested CE with EcoRI (10uL DNA).
Yesterdays gel:
| Well | Part | Expected Plasmid Size | Status |
| 1 | |||
| 2 | |||
| 3 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✔ | |
| 4 | E0240 | 876 | ✔ |
| 5 | B0031 | 14 | ? |
| 6 | J04630 | 857 | ✔ |
| 7 | R0010 | 200 | ✔ |
| 8 | R0040 | 54 | too small |
| 9 | pSB1C3 | 2072 | ✔ |
| 10 | pSB1AK3 | 3426 | ✔ |
| 11 | Assembly 1 | ||
| 12 | Assembly 3 | ||
| 13 | Assembly 5 | ||
| 14 | Assembly 6 | ||
| 15 | Assembly 7 | ||
| 16 | Assembly 8 | ||
| 17 | trbK X+P | ✔ | |
| 18 | GFP-gen E+S | ✔ | |
| 19 | pTet+RBS E+S | ? | |
| 20 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✔ |
Made 12 plates. 4 High KAN. 4 Low KAN. 4 TRI.
Placed 50uL of overnight R751 + pKD46 culture into 4 x 5 mL tubes.
In 30C incubator at 11:15
11:45 0.034 OD
12:30 0.065 OD
13:17 0.126 OD
Induced +L-arabinose tubes with L-arabinose (40uL 1M stock added to 4mL). Control tube has no L-arabinose added.
In 30C incubator at 13:25
14:40 0.497 OD
on Ice at 14:57
centrifuge 1 @ 15:13 - 10 min 4000 rcf 3C
centrifuge 2 @ 15:44 - 10 min 4000 rcf 3C
centrifuge 3 @ 16:10 - 10 min 4000 rcf 3C
12 plates made, of which 8 are various controls
| Plate ID | Knockout | Variables | AB |
| 1H | oriTR_KO | +L-arabinose +PCR | 1xKAN |
| 1L | oriTR_KO | +L-arabinose +PCR | 0.5xKAN |
| 2 | oriTR_KO | +L-arabinose -PCR | TRI |
| 3H | oriTR_KO | -L-arabinose +PCR | 1xKAN |
| 3L | oriTR_KO | -L-arabinose +PCR | 0.5xKAN |
| 4 | oriTR_KO | -L-arabinose -PCR | TRI |
| 5H | trbK_KO | +L-arabinose +PCR | 1xKAN |
| 5L | trbK_KO | +L-arabinose +PCR | 0.5xKAN |
| 6 | trbK_KO | +L-arabinose -PCR | TRI |
| 7H | trbK_KO | -L-arabinose +PCR | 1xKAN |
| 7L | trbK_KO | -L-arabinose +PCR | 0.5xKAN |
| 8 | trbK_KO | -L-arabinose -PCR | TRI |
Tim Vos helped with electroporation step. Electroporator was set to 600 ohm.
| Incubator Time | Knockout | Variables | Time Constant |
| 18:42 | oriTR_KO | +L-arabinose +PCR | 12.0 |
| 18:45 | oriTR_KO | +L-arabinose -PCR | 13.3 |
| 18:48 | oriTR_KO | -L-arabinose +PCR | 10.4 |
| 18:50 | oriTR_KO | -L-arabinose -PCR | 13.4 |
| 18:54 | trbK_KO | +L-arabinose +PCR | 12.0 |
| 18:56 | trbK_KO | +L-arabinose -PCR | 12.9 |
| 17:02 | trbK_KO | -L-arabinose +PCR | 13.0 |
| 17:05 | trbK_KO | -L-arabinose -PCR | 12.6 |
After one hour 200 uL was plated. oriTR_KO +L-ara +PCR and trbK_KO +L-ara +PCR placed in fridge at 18:30.
Made 5mL tube cultures for assemblies CB and CC.
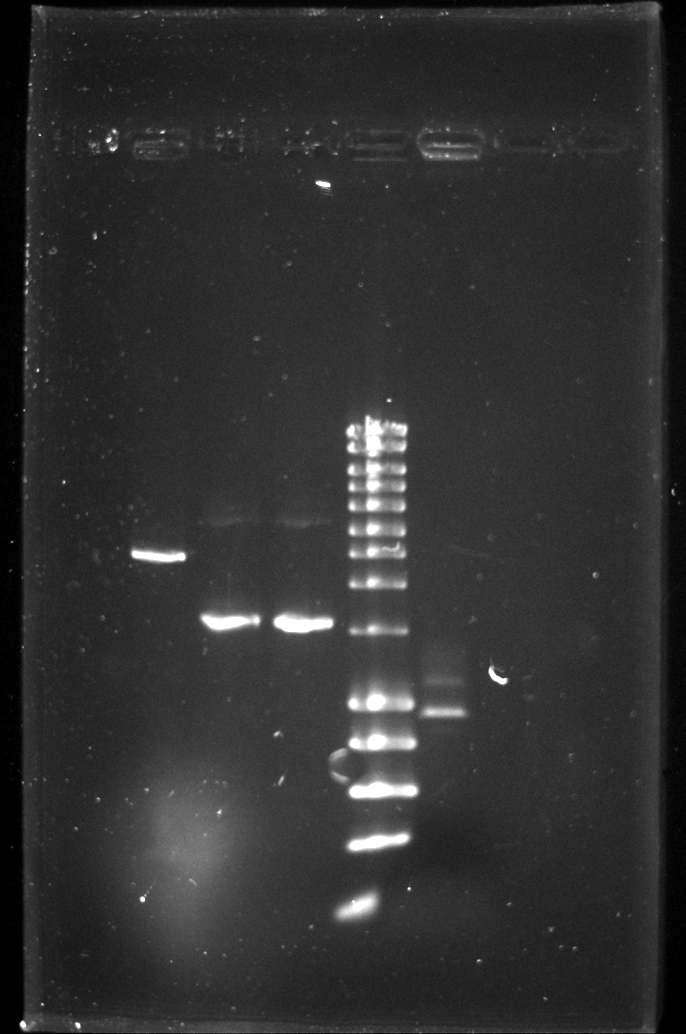
Todays 8 well gel to check the linear fragments for the knockout:
| Well | Part | Expected Plasmid Size | Status |
| 2 | CE | 2157 | ✔ |
| 3 | oriTR_KO_PCR | 1596 | ✔ |
| 4 | trbK_KO_PCR | 1596 | ✔ |
| 5 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✔ | |
| 6 | ? | ? |
Daniel
Yesterday´s gel confirm the sizes of all the biobricks needed (see the wells 4-10 in the first gel of this site), I did a second round of digestions though, in order to have enough DNA. After a new gel and check sizes, I did the assemblies 1A and 2A (see locks and keys section). Also, I transformed by heat shock and electroporation these assemblies and did plates and culture tubes with respective antibiotic.
 "
"