Team:TUDelft/18 August 2009
Lab Notebook
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
18 August 2009
Sriram
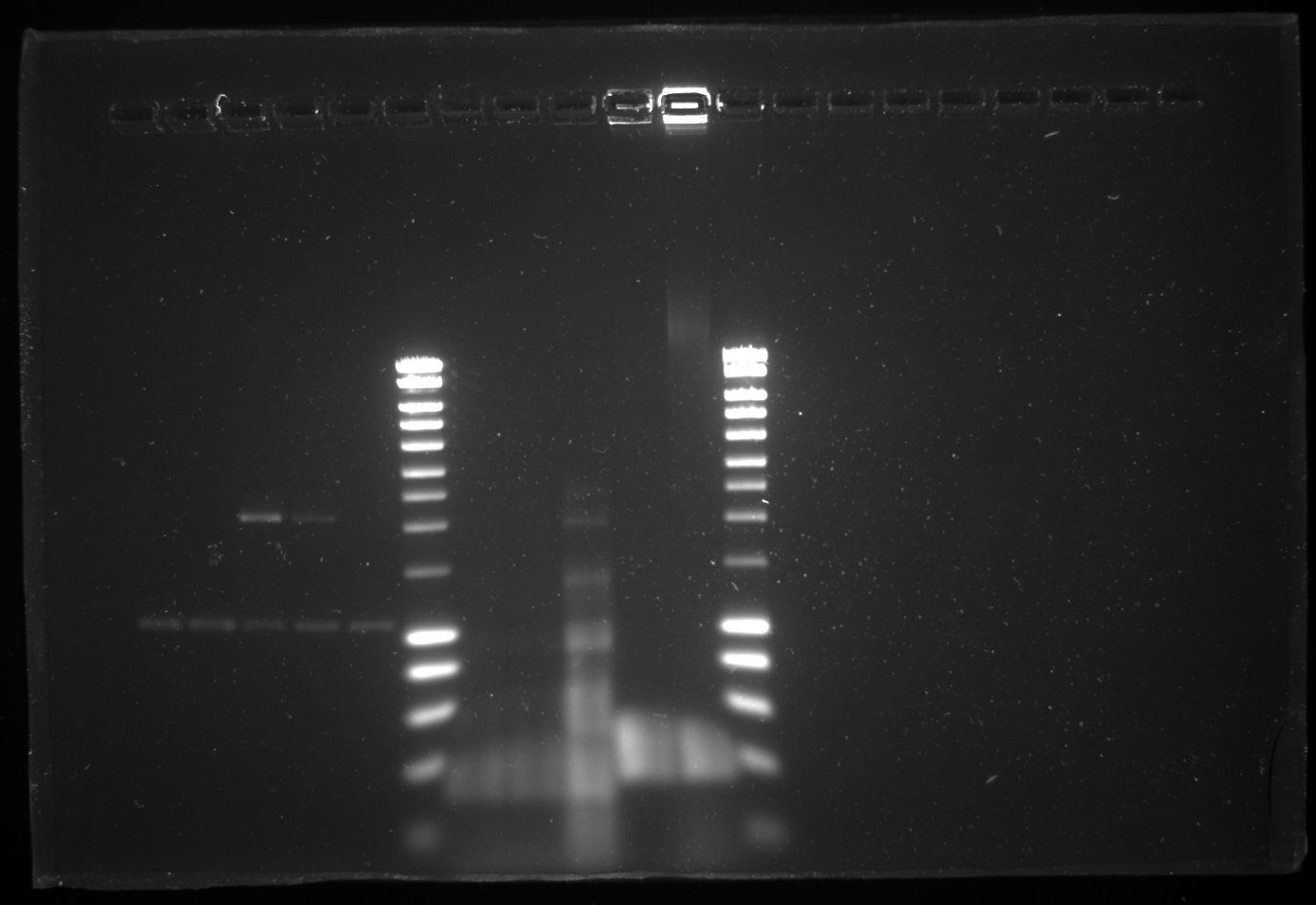
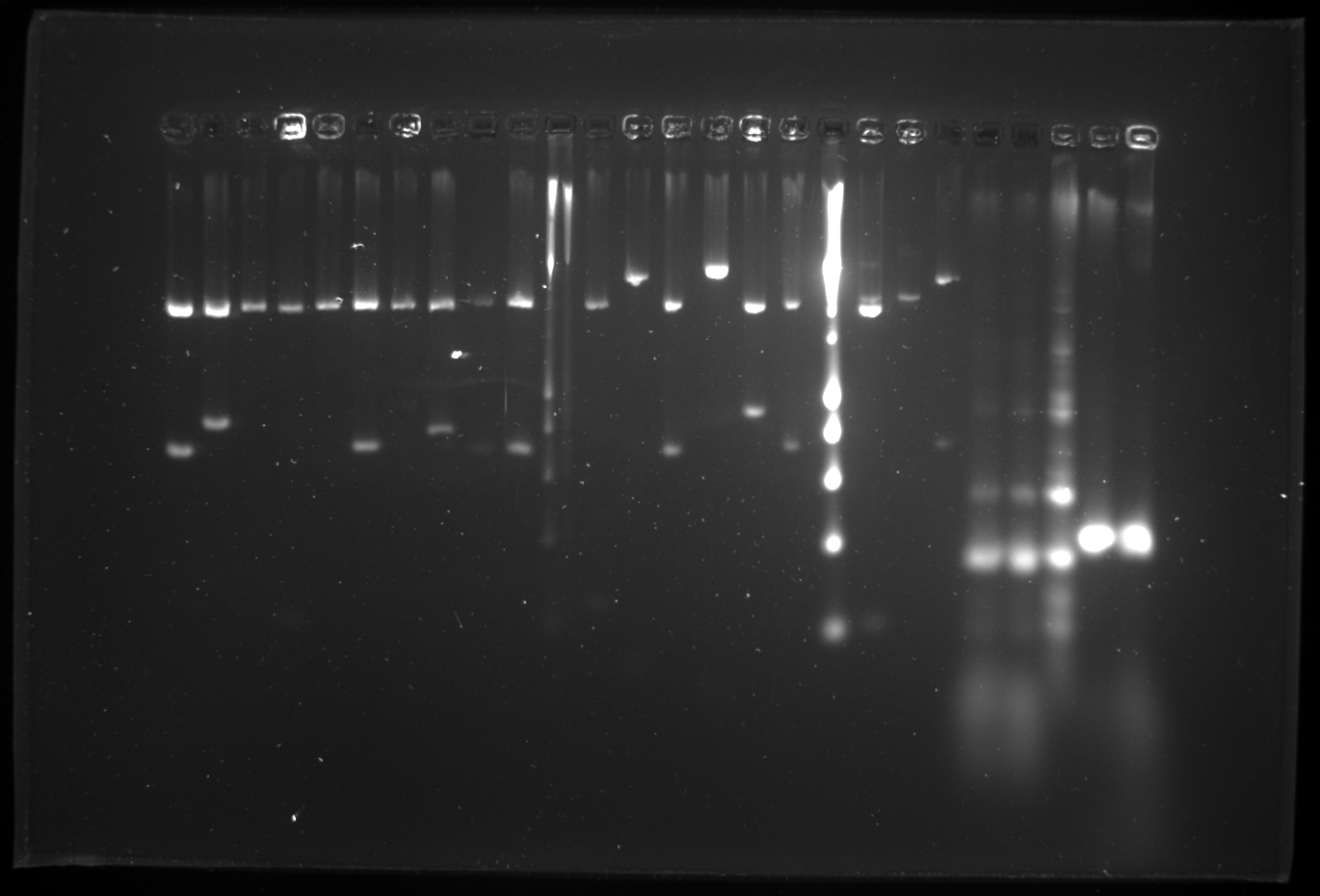
Checking the yesterday's gel (shown below) I found that since the ladder didn't run well we couldn't interpret the size properly. hence I decided to do the whole assembly again. Or else we need to follow the back up plan for negative cascade alone created by Daniel. So today to start everything again I made cultures for all the primary biobricks to start the assembly all over again. We also have one problem the restriction enzyme SpeI is getting over. So it was ordered today.
Calin
Yesterdays 20 well 1% gel:
| Well | Part | Expected Plasmid Size | Status |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✔ | |
| 7 | Colony PCR 1A - R751ΔtrbK | 1616 | ✖ |
| 8 | Colony PCR 1A - R751ΔtrbK | 1616 | ✖ |
| 9 | Colony PCR R751 + pKD46 + trbK_KO_mod_S | 348 | ✔ |
| 10 | Colony PCR R751ΔoriTR -L-ara +PCR control | ? | |
| 11 | Colony PCR R751 + pKD46 + oriTR_KO_mod_S | 398 | ✔ |
| 12 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✔ |
Yesterdays 26 well 2% gel:
| Well | Part | Expected Plasmid Size | Status |
| 1 | pTet | ||
| 2 | RBS-cI-RBS | ||
| 3 | pSB1C3 | ||
| 4 | pLacI | ||
| 5 | RBS | ||
| 6 | pSB1C3 | ||
| 7 | pTet | ||
| 8 | lock3c | ||
| 9 | pSB1C3 (not loaded well) | ||
| 10 | pSB1C3 | ||
| 11 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✖ | |
| 12 | cI | ||
| 13 | Double Terminator | ||
| 14 | pSB1C3 | ||
| 15 | λp-in | ||
| 16 | RBS-GFP-Double Term | ||
| 17 | pSB1C3 | ||
| 18 | [http://www.eurogentec.com/EGT/Images/RESALES/Electrophoresis/Regular%20DNA%20Ladder/7-SmartLadder.jpg DNA Ladder] | ✖ | |
| 19 | pLacI | ||
| 20 | key3c | ||
| 21 | pSB1AK3 | ||
| 22 | Colony PCR 1A - R751ΔtrbK | 1616 | ✖ |
| 23 | Colony PCR 1A - R751ΔtrbK | 1616 | ✖ |
| 24 | Colony PCR R751 + pKD46 + trbK_KO_mod_S | 348 | ✔ |
| 25 | Colony PCR R751ΔoriTR -L-ara +PCR control | ? | |
| 26 | Colony PCR R751 + pKD46 + oriTR_KO_mod_S | 398 | ✔ |
For the trbK conjugation test 24 plates were made:
| Set 1 | |||
| Plate ID | Antibiotics | Dilution | |
| D1tr | TRI | 100 | |
| D2tr | TRI | 10-1 | |
| D3tr | TRI | 10-2 | |
| D4tr | TRI | 10-3 | |
| D5tr | TRI | 10-4 | |
| T1tr | TRI + CAM | 100 | |
| T2tr | TRI + CAM | 10-1 | |
| T3tr | TRI + CAM | 10-2 | |
| T4tr | TRI + CAM | 10-3 | |
| T5tr | TRI + CAM | 10-4 | |
| R1tr | CAM | 100 | |
| R2tr | CAM | 10-1 |
| Set 2 | |||
| Plate ID | Antibiotics | Dilution | |
| D6tr | TRI | 100 | |
| D7tr | TRI | 10-1 | |
| D8tr | TRI | 10-2 | |
| D9tr | TRI | 10-3 | |
| D10tr | TRI | 10-4 | |
| T6tr | TRI + CAM | 100 | |
| T7tr | TRI + CAM | 10-1 | |
| T8tr | TRI + CAM | 10-2 | |
| T9tr | TRI + CAM | 10-3 | |
| T10tr | TRI + CAM | 10-4 | |
| R3tr | CAM | 100 | |
| R4tr | CAM | 10-1 |
For the oriTR conjugation test 51 plates were made:
| Set 1 | |||
| Plate ID | Antibiotics | Dilution | |
| D1ori | TRI + CAM | 100 | |
| D2ori | TRI + CAM | 10-1 | |
| D3ori | TRI + CAM | 10-2 | |
| D4ori | TRI + CAM | 10-3 | |
| D5ori | TRI + CAM | 10-4 | |
| Ts1ori | AMP + CAM | 100 | |
| Ts2ori | AMP + CAM | 10-1 | |
| Ts3ori | AMP + CAM | 10-2 | |
| Ts4ori | AMP + CAM | 10-3 | |
| Ts5ori | AMP + CAM | 10-4 | |
| TL1ori | TRI + AMP | 100 | |
| TL2ori | TRI + AMP | 10-1 | |
| TL3ori | TRI + AMP | 10-2 | |
| TL4ori | TRI + AMP | 10-3 | |
| TL5ori | TRI + AMP | 10-4 | |
| R1ori | AMP | 100 | |
| R2ori | AMP | 10-1 |
| Set 2 | |||
| Plate ID | Antibiotics | Dilution | |
| D6ori | TRI + CAM | 100 | |
| D7ori | TRI + CAM | 10-1 | |
| D8ori | TRI + CAM | 10-2 | |
| D9ori | TRI + CAM | 10-3 | |
| D10ori | TRI + CAM | 10-4 | |
| Ts6ori | AMP + CAM | 100 | |
| Ts7ori | AMP + CAM | 10-1 | |
| Ts8ori | AMP + CAM | 10-2 | |
| Ts9ori | AMP + CAM | 10-3 | |
| Ts10ori | AMP + CAM | 10-4 | |
| TL6ori | TRI + AMP | 100 | |
| TL7ori | TRI + AMP | 10-1 | |
| TL8ori | TRI + AMP | 10-2 | |
| TL9ori | TRI + AMP | 10-3 | |
| TL10ori | TRI + AMP | 10-4 | |
| R3ori | AMP | 100 | |
| R4ori | AMP | 10-1 |
| Set 3 | |||
| Plate ID | Antibiotics | Dilution | |
| D11ori | TRI + CAM | 100 | |
| D12ori | TRI + CAM | 10-1 | |
| D13ori | TRI + CAM | 10-2 | |
| D14ori | TRI + CAM | 10-3 | |
| D15ori | TRI + CAM | 10-4 | |
| Ts11ori | AMP + CAM | 100 | |
| Ts12ori | AMP + CAM | 10-1 | |
| Ts13ori | AMP + CAM | 10-2 | |
| Ts14ori | AMP + CAM | 10-3 | |
| Ts15ori | AMP + CAM | 10-4 | |
| TL11ori | TRI + AMP | 100 | |
| TL12ori | TRI + AMP | 10-1 | |
| TL13ori | TRI + AMP | 10-2 | |
| TL14ori | TRI + AMP | 10-3 | |
| TL15ori | TRI + AMP | 10-4 | |
| R5ori | AMP | 100 | |
| R6ori | AMP | 10-1 |
Tim Weenink
Results of the 24h sequencing are in:
- !BPEA1 (R) correct in reliable part of sequencing file
- !BCEC1 (R) correct in reliable part of sequencing file
- !CCEA1 (F) perfect
- !CCEC2 (F) perfect
- !SPEA1 (F) Assebly with !A and BBa_K142202. !A is not supposed to be there
- !SPEA1 (R) Assebly with !A and BBa_K142202. !A is not supposed to be there
- !SPEC1 (F) Failed (as expected)
- !SPEC1 (R) Failed (as expected)
- *I6F (F) Perfect
- *S3 (F) Is not BBa_K142205, but in fact BBa_K142203
- *S3 (R) Is not BBa_K142205, but in fact BBa_K142203
Daniel
Growth, miniprep:
| Biobrick | [DNA] ng/uL |
| C0051 | 45 |
| P0440 | 48.2 |
| R0051 | 22.8 |
| I13504 | 70.1 |
| Ca | 28.1 |
 "
"